
I've been asked before what's the difference between soap and detergent. After giving my proof-readers a little taste of chemistry, I noticed their eyes began to look like doughnuts... glazed. To hopefully make things more understandable, I have included pictures that my kids I drew. OK... yes, I drew them.
Surfactants
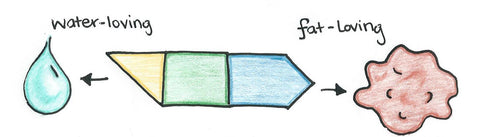
Both soaps and detergents are known as surfactants (short for surface-active agents). Surfactant molecules contain a lipophilic (fat-loving) end that attaches grease dirt and a hydrophilic (water-loving) end which makes the molecule dissolve in water. The electrical charge on the water-loving end of the molecule distinguishes between the different types of surfactants. Surfactants come in four different types: anionic, nonionic, cationic, and amphoteric.
Soap
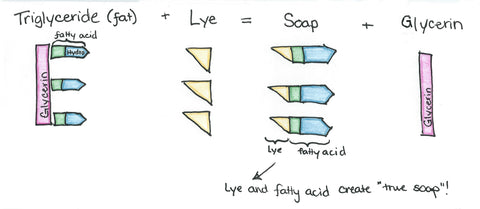
Soap, by definition, is the product of a fat and an alkali -- usually sodium or potassium hydroxide. Fat, made of triglycerides, is composed of three fatty acids (hence the prefix "tri-") connected to one glycerin (hence "glyceride"). There are many types of triglycerides; each type consists of its own particular combination of fatty acids.

It is the fatty acid part that reacts with sodium or potassium hydroxide that creates a soap with the glycerin left behind.
Detergent
Detergents are created in a multi-step process -- making a completely synthetic substance. It's literally called a "slurry mixture" when being developed.
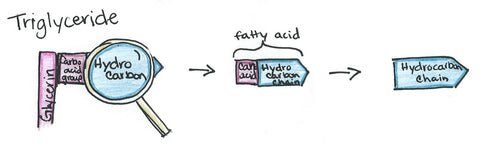
It contains a hydrocarbon (which is a fraction of a fatty acid, which is a part of a triglyceride/whole fat) from either a petrochemical, derived from petroleum, or an oleochemical, derived from fat and oils. The hydrocarbon will become fat-loving end of the surfactant.
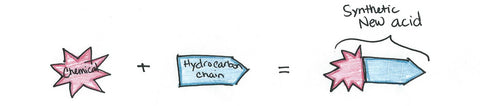
Other chemicals, such as sulfur trioxide, sulfuric acid, and ethylene oxide, are used to produce the water-loving end of the surfactant molecule. The hydrocarbon plus the other chemicals creates the new, synthetic acid which is ready to be mixed with another agent.
Sometimes it is mixed with ethylene oxide to create a nonionic surfactant. Other times it is mixed with an alkali - usually sodium or potassium hydroxide - to make an anionic surfactant. However, the differences in detergent ions is going beyond the scope of this post.
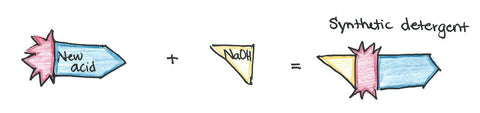
I'd like to point out that the creation of detergents does not include glycerin which is why we use conditioner after shampoo and lotion after body wash. They not only remove oils which are our protective barrier, but also fail to moisturize (via glycerin) nor leave a moisturizing barrier behind. Additionally, the Journal of the American College of Toxicology (1983, Vol. 2, No. 7), has noted that detergents are skin irritants as well.
In labeling, detergents may not be called soap. It does not contain a full fatty acid (only a hydrocarbon) and it may or may not even use an alkali. It's completely synthetic. It does not fit the definition of a soap; it's a detergent. However, this doesn't stop clever marketers from calling their product "beauty bar," "anti-aging moisturizing bar," or "deodorant bar." This also doesn't stop marketers for wanna-be-natural products to say their sodium lauryl sulfate is all-natural because the hydrocarbon came "from coconut oil."
Often times people use the word "soap" when referring to anything that cleans -- for example, "dish soap," "hand soap," "anti-bacterial soap," etc. However, this can be detrimental for one's understanding because, unlike real soap which mechanically removes dirt AND kills bacteria, detergents can only physically remove dirt. You can learn more about detergents in my next post!
Thanks for reading!




https://www.acerthailnd.com/
https://www.acerthailnd.com/huaythai
https://www.acerthailnd.com/contact
https://github.com/turingaicloud/quickstart/issues/10
https://github.com/kvspb/nginx-auth-ldap/issues/259
https://github.com/Kr1s77/FgSurfing/issues/2
https://github.com/stawel/ht301_hacklib/issues/17
https://sistacafe.com/summaries/preview/219483
https://sistacafe.com/summaries/preview/219515
https://sistacafe.com/summaries/preview/219516
https://sistacafe.com/summaries/preview/219517
https://sistacafe.com/summaries/preview/219522
https://sistacafe.com/summaries/preview/219527
https://sistacafe.com/summaries/preview/219528
https://sistacafe.com/summaries/preview/219529
https://sistacafe.com/summaries/preview/219530
https://sistacafe.com/summaries/preview/219531
https://sistacafe.com/summaries/preview/219532
https://sistacafe.com/summaries/preview/219534
https://sistacafe.com/summaries/preview/219537
https://sistacafe.com/summaries/preview/219539
https://sistacafe.com/summaries/preview/219540
https://sistacafe.com/summaries/preview/219544
https://sistacafe.com/summaries/preview/219545
https://sistacafe.com/summaries/preview/219546
https://sistacafe.com/summaries/preview/219547
https://sistacafe.com/summaries/preview/219548
https://sistacafe.com/summaries/preview/219549
https://sistacafe.com/summaries/preview/219550
https://sistacafe.com/summaries/preview/219551
https://sistacafe.com/summaries/preview/219560
https://sistacafe.com/summaries/preview/219561
https://sistacafe.com/summaries/preview/219562
https://sistacafe.com/summaries/preview/219574
https://sistacafe.com/summaries/preview/219580
https://sistacafe.com/summaries/preview/219583
https://sistacafe.com/summaries/preview/219585
https://sistacafe.com/summaries/preview/219486
https://sistacafe.com/summaries/preview/219484
https://sistacafe.com/summaries/preview/219490
https://sistacafe.com/summaries/preview/219496
https://sistacafe.com/summaries/preview/219491
https://sistacafe.com/summaries/preview/219492
https://sistacafe.com/summaries/preview/219495
https://sistacafe.com/summaries/preview/219494
https://sistacafe.com/summaries/preview/219497
https://sistacafe.com/summaries/preview/219498
https://sistacafe.com/summaries/preview/219499
https://sistacafe.com/summaries/preview/219493
https://sistacafe.com/summaries/preview/219500
https://sistacafe.com/summaries/preview/219501
https://sistacafe.com/summaries/preview/219502
https://sistacafe.com/summaries/preview/219503
https://sistacafe.com/summaries/preview/219504
https://sistacafe.com/summaries/preview/219505
https://sistacafe.com/summaries/preview/219506
https://sistacafe.com/summaries/preview/219507
https://sistacafe.com/summaries/preview/219519
https://sistacafe.com/summaries/preview/219518
https://sistacafe.com/summaries/preview/219520
https://sistacafe.com/summaries/preview/219523
https://sistacafe.com/summaries/preview/219526
https://sistacafe.com/summaries/preview/219524
https://sistacafe.com/summaries/preview/219525
https://sistacafe.com/summaries/preview/219521
https://sistacafe.com/summaries/preview/219536
https://sistacafe.com/summaries/preview/219555
https://sistacafe.com/summaries/preview/219576
https://sistacafe.com/summaries/preview/219579
https://sistacafe.com/summaries/preview/219556
https://sistacafe.com/summaries/preview/219558
https://sistacafe.com/summaries/preview/219557
https://sistacafe.com/summaries/preview/219559
https://sistacafe.com/summaries/preview/219564
https://sistacafe.com/summaries/preview/219565
https://sistacafe.com/summaries/preview/219566
https://sistacafe.com/summaries/preview/219570
https://sistacafe.com/summaries/preview/219568
https://sistacafe.com/summaries/preview/219575
https://sistacafe.com/summaries/preview/219577
https://sistacafe.com/summaries/preview/219582
https://sistacafe.com/summaries/preview/219581
https://sistacafe.com/summaries/preview/219567
https://sistacafe.com/summaries/preview/219578
https://sistacafe.com/summaries/preview/219571
https://sistacafe.com/summaries/preview/219569
https://sistacafe.com/summaries/preview/219589
https://sistacafe.com/summaries/preview/219593
https://sistacafe.com/summaries/preview/219595
https://sistacafe.com/summaries/preview/219619
https://sistacafe.com/summaries/preview/219620
https://sistacafe.com/summaries/preview/219621
https://sistacafe.com/summaries/preview/219622
https://sistacafe.com/summaries/preview/219623
https://sistacafe.com/summaries/preview/219624
https://sistacafe.com/summaries/preview/219625
https://sistacafe.com/summaries/preview/219626
https://sistacafe.com/summaries/preview/219627
https://sistacafe.com/summaries/preview/219628
https://sistacafe.com/summaries/preview/219629
https://sistacafe.com/summaries/preview/219630
https://sistacafe.com/summaries/preview/219631
https://sistacafe.com/summaries/preview/219632
https://sistacafe.com/summaries/preview/219633
https://sistacafe.com/summaries/preview/219634
https://sistacafe.com/summaries/preview/219635
https://sistacafe.com/summaries/preview/219637
https://sistacafe.com/summaries/preview/219638
https://sistacafe.com/summaries/preview/219639
https://sistacafe.com/summaries/preview/219640
https://sistacafe.com/summaries/preview/219641
https://sistacafe.com/summaries/preview/219642
https://sistacafe.com/summaries/preview/219645
https://sistacafe.com/summaries/preview/219659
https://sistacafe.com/summaries/preview/219660
https://sistacafe.com/summaries/preview/219666
https://sistacafe.com/summaries/preview/219667
https://sistacafe.com/summaries/preview/219668
https://sistacafe.com/summaries/preview/219669
https://sistacafe.com/summaries/preview/219670
https://sistacafe.com/summaries/preview/219604
https://sistacafe.com/summaries/preview/219605
https://sistacafe.com/summaries/preview/219606
https://sistacafe.com/summaries/preview/219610
https://sistacafe.com/summaries/preview/219608
https://sistacafe.com/summaries/preview/219607
https://sistacafe.com/summaries/preview/219612
https://sistacafe.com/summaries/preview/219609
https://sistacafe.com/summaries/preview/219611
https://sistacafe.com/summaries/preview/219613
https://sistacafe.com/summaries/preview/219614
https://sistacafe.com/summaries/preview/219618
https://sistacafe.com/summaries/preview/219616
https://sistacafe.com/summaries/preview/219617
https://sistacafe.com/summaries/preview/219615
https://sistacafe.com/summaries/preview/219654
https://sistacafe.com/summaries/preview/219651
https://sistacafe.com/summaries/preview/219653
https://sistacafe.com/summaries/preview/219650
https://sistacafe.com/summaries/preview/219657
https://sistacafe.com/summaries/preview/219658
https://sistacafe.com/summaries/preview/219652
https://sistacafe.com/summaries/preview/219655
https://sistacafe.com/summaries/preview/219663
https://sistacafe.com/summaries/preview/219665
https://sistacafe.com/summaries/preview/219662
https://sistacafe.com/summaries/preview/219661
https://sistacafe.com/summaries/preview/219649
https://sistacafe.com/summaries/preview/219664
https://sistacafe.com/summaries/preview/219656
https://sistacafe.com/summaries/preview/219646
https://sistacafe.com/summaries/preview/219644
https://sistacafe.com/summaries/preview/219647
https://sistacafe.com/summaries/preview/219671
https://sistacafe.com/summaries/preview/219672
https://sistacafe.com/summaries/preview/219673
https://sistacafe.com/summaries/preview/219674
https://sistacafe.com/summaries/preview/219675
https://sistacafe.com/summaries/preview/219676
https://sistacafe.com/summaries/preview/219677
https://sistacafe.com/summaries/preview/219678
https://sistacafe.com/summaries/preview/219679
https://sistacafe.com/summaries/preview/219680
https://sistacafe.com/summaries/preview/219681
https://sistacafe.com/summaries/preview/219682
https://sistacafe.com/summaries/preview/219683
https://sistacafe.com/summaries/preview/219684
https://sistacafe.com/summaries/preview/219685
https://sistacafe.com/summaries/preview/219686
https://sistacafe.com/summaries/preview/219687
https://sistacafe.com/summaries/preview/219688
https://sistacafe.com/summaries/preview/219689
https://sistacafe.com/summaries/preview/219690
https://sistacafe.com/summaries/preview/219691
https://sistacafe.com/summaries/preview/219692
https://sistacafe.com/summaries/preview/219693
https://sistacafe.com/summaries/preview/219694
https://sistacafe.com/summaries/preview/219695
https://sistacafe.com/summaries/preview/219696
https://sistacafe.com/summaries/preview/219697
https://sistacafe.com/summaries/preview/219698
https://sistacafe.com/summaries/preview/219699
https://sistacafe.com/summaries/preview/219700
https://sistacafe.com/summaries/preview/219701
https://sistacafe.com/summaries/preview/219702
https://sistacafe.com/summaries/preview/219709
https://sistacafe.com/summaries/preview/219710
https://sistacafe.com/summaries/preview/219711
https://sistacafe.com/summaries/preview/219712
https://sistacafe.com/summaries/preview/219713
https://sistacafe.com/summaries/preview/219714
https://sistacafe.com/summaries/preview/219715
https://sistacafe.com/summaries/preview/219716
https://sistacafe.com/summaries/preview/219717
https://sistacafe.com/summaries/preview/219718
https://sistacafe.com/summaries/preview/219719
https://sistacafe.com/summaries/preview/219720
https://sistacafe.com/summaries/preview/219721
https://sistacafe.com/summaries/preview/219722
https://sistacafe.com/summaries/preview/219723
https://sistacafe.com/summaries/preview/219724
https://sistacafe.com/summaries/preview/219725
https://sistacafe.com/summaries/preview/219726
https://sistacafe.com/summaries/preview/219727
https://sistacafe.com/summaries/preview/219729
https://sistacafe.com/summaries/preview/219730
https://sistacafe.com/summaries/preview/219731
https://sistacafe.com/summaries/preview/219740
https://sistacafe.com/summaries/preview/219741
https://sistacafe.com/summaries/preview/219742
https://sistacafe.com/summaries/preview/219743
https://sistacafe.com/summaries/preview/219750
https://sistacafe.com/summaries/preview/219751
https://sistacafe.com/summaries/preview/219752
https://sistacafe.com/summaries/preview/219753
https://sistacafe.com/summaries/preview/219703
https://sistacafe.com/summaries/preview/219705
https://sistacafe.com/summaries/preview/219707
https://sistacafe.com/summaries/preview/219708
https://sistacafe.com/summaries/preview/219706
https://sistacafe.com/summaries/preview/219732
https://sistacafe.com/summaries/preview/219704
https://sistacafe.com/summaries/preview/219733
https://sistacafe.com/summaries/preview/219734
https://sistacafe.com/summaries/preview/219735
https://sistacafe.com/summaries/preview/219737
https://sistacafe.com/summaries/preview/219739
https://sistacafe.com/summaries/preview/219738
https://sistacafe.com/summaries/preview/219736
https://sistacafe.com/summaries/preview/219744
https://sistacafe.com/summaries/preview/219745
https://sistacafe.com/summaries/preview/219748
https://sistacafe.com/summaries/preview/219746
https://sistacafe.com/summaries/preview/219749
https://sistacafe.com/summaries/preview/219747
https://sistacafe.com/summaries/preview/219761
https://sistacafe.com/summaries/preview/219763
https://sistacafe.com/summaries/preview/219762
https://sistacafe.com/summaries/preview/219760
https://sistacafe.com/summaries/preview/219756
https://sistacafe.com/summaries/preview/219755
https://sistacafe.com/summaries/preview/219754
https://sistacafe.com/summaries/preview/219758
https://sistacafe.com/summaries/preview/219757
https://sistacafe.com/summaries/preview/219759
https://sistacafe.com/summaries/preview/219765
https://sistacafe.com/summaries/preview/219766
https://sistacafe.com/summaries/preview/219767
https://sistacafe.com/summaries/preview/219768
https://sistacafe.com/summaries/preview/219769
https://sistacafe.com/summaries/preview/219770
https://sistacafe.com/summaries/preview/219771
https://sistacafe.com/summaries/preview/219772
https://sistacafe.com/summaries/preview/219773
https://sistacafe.com/summaries/preview/219774
https://sistacafe.com/summaries/preview/219775
https://sistacafe.com/summaries/preview/219776
https://sistacafe.com/summaries/preview/219777
https://sistacafe.com/summaries/preview/219778
https://sistacafe.com/summaries/preview/219779
https://sistacafe.com/summaries/preview/219780
https://sistacafe.com/summaries/preview/219781
https://sistacafe.com/summaries/preview/219782
https://sistacafe.com/summaries/preview/219783
https://sistacafe.com/summaries/preview/219784
https://sistacafe.com/summaries/preview/219785
https://sistacafe.com/summaries/preview/219786
https://sistacafe.com/summaries/preview/219787
https://sistacafe.com/summaries/preview/219788
https://sistacafe.com/summaries/preview/219789
https://sistacafe.com/summaries/preview/219790
https://sistacafe.com/summaries/preview/219791
https://sistacafe.com/summaries/preview/219792
https://sistacafe.com/summaries/preview/219793
https://sistacafe.com/summaries/preview/219794
https://sistacafe.com/summaries/preview/219795
https://sistacafe.com/summaries/preview/219796
https://sistacafe.com/summaries/preview/219797
https://sistacafe.com/summaries/preview/219806
https://sistacafe.com/summaries/preview/219807
https://sistacafe.com/summaries/preview/219808
https://sistacafe.com/summaries/preview/219809
https://sistacafe.com/summaries/preview/219810
https://sistacafe.com/summaries/preview/219811
https://sistacafe.com/summaries/preview/219812
https://sistacafe.com/summaries/preview/219813
https://sistacafe.com/summaries/preview/219814
https://sistacafe.com/summaries/preview/219815
https://sistacafe.com/summaries/preview/219816
https://sistacafe.com/summaries/preview/219817
https://sistacafe.com/summaries/preview/219818
https://sistacafe.com/summaries/preview/219819
https://sistacafe.com/summaries/preview/219820
https://sistacafe.com/summaries/preview/219822
https://sistacafe.com/summaries/preview/219823
https://sistacafe.com/summaries/preview/219824
https://sistacafe.com/summaries/preview/219825
https://sistacafe.com/summaries/preview/219826
https://sistacafe.com/summaries/preview/219878
https://sistacafe.com/summaries/preview/219880
https://sistacafe.com/summaries/preview/219881
https://sistacafe.com/summaries/preview/219885
https://sistacafe.com/summaries/preview/219886
https://sistacafe.com/summaries/preview/219890
https://sistacafe.com/summaries/preview/219891
https://sistacafe.com/summaries/preview/219892
https://sistacafe.com/summaries/preview/219893
https://sistacafe.com/summaries/preview/219894
https://sistacafe.com/summaries/preview/219803
https://sistacafe.com/summaries/preview/219800
https://sistacafe.com/summaries/preview/219801
https://sistacafe.com/summaries/preview/219802
https://sistacafe.com/summaries/preview/219828
https://sistacafe.com/summaries/preview/219827
https://sistacafe.com/summaries/preview/219805
https://sistacafe.com/summaries/preview/219834
https://sistacafe.com/summaries/preview/219833
https://sistacafe.com/summaries/preview/219829
https://sistacafe.com/summaries/preview/219804
https://sistacafe.com/summaries/preview/219831
https://sistacafe.com/summaries/preview/219839
https://sistacafe.com/summaries/preview/219842
https://sistacafe.com/summaries/preview/219836
https://sistacafe.com/summaries/preview/219832
https://sistacafe.com/summaries/preview/219837
https://sistacafe.com/summaries/preview/219838
https://sistacafe.com/summaries/preview/219841
https://sistacafe.com/summaries/preview/219835
https://sistacafe.com/summaries/preview/219843
https://sistacafe.com/summaries/preview/219845
https://sistacafe.com/summaries/preview/219844
https://sistacafe.com/summaries/preview/219847
https://sistacafe.com/summaries/preview/219846
https://sistacafe.com/summaries/preview/219848
https://sistacafe.com/summaries/preview/219830
https://sistacafe.com/summaries/preview/219849
https://sistacafe.com/summaries/preview/219850
https://sistacafe.com/summaries/preview/219851
https://sistacafe.com/summaries/preview/219852
https://sistacafe.com/summaries/preview/219853
https://sistacafe.com/summaries/preview/219854
https://sistacafe.com/summaries/preview/219855
https://sistacafe.com/summaries/preview/219856
https://sistacafe.com/summaries/preview/219857
https://sistacafe.com/summaries/preview/219858
https://sistacafe.com/summaries/preview/219859
https://sistacafe.com/summaries/preview/219860
https://sistacafe.com/summaries/preview/219861
https://sistacafe.com/summaries/preview/219862
https://sistacafe.com/summaries/preview/219863
https://sistacafe.com/summaries/preview/219864
https://sistacafe.com/summaries/preview/219865
https://sistacafe.com/summaries/preview/219866
https://sistacafe.com/summaries/preview/219867
https://sistacafe.com/summaries/preview/219868
https://sistacafe.com/summaries/preview/219869
https://sistacafe.com/summaries/preview/219870
https://sistacafe.com/summaries/preview/219871
https://sistacafe.com/summaries/preview/219872
https://sistacafe.com/summaries/preview/219873
https://sistacafe.com/summaries/preview/219874
https://sistacafe.com/summaries/preview/219875
https://sistacafe.com/summaries/preview/219876
https://sistacafe.com/summaries/preview/219877
https://sistacafe.com/summaries/preview/219879
https://sistacafe.com/summaries/preview/219882
https://sistacafe.com/summaries/preview/219883
https://sistacafe.com/summaries/preview/219884
https://sistacafe.com/summaries/preview/219887
https://sistacafe.com/summaries/preview/219888
https://sistacafe.com/summaries/preview/219889
https://sistacafe.com/summaries/preview/219896
https://sistacafe.com/summaries/preview/219897
https://sistacafe.com/summaries/preview/219898
https://sistacafe.com/summaries/preview/219899
https://sistacafe.com/summaries/preview/219900
https://sistacafe.com/summaries/preview/219901
https://sistacafe.com/summaries/preview/219902
https://sistacafe.com/summaries/preview/219903
https://sistacafe.com/summaries/preview/219904
https://sistacafe.com/summaries/preview/219905
https://sistacafe.com/summaries/preview/219906
https://sistacafe.com/summaries/preview/219907
https://sistacafe.com/summaries/preview/219908
https://sistacafe.com/summaries/preview/219909
https://sistacafe.com/summaries/preview/219910
https://sistacafe.com/summaries/preview/219911
https://sistacafe.com/summaries/preview/219912
https://sistacafe.com/summaries/preview/219913
https://sistacafe.com/summaries/preview/219914
https://sistacafe.com/summaries/preview/219915
https://sistacafe.com/summaries/preview/219916
https://sistacafe.com/summaries/preview/219917
https://sistacafe.com/summaries/preview/219918
https://sistacafe.com/summaries/preview/219919
https://sistacafe.com/summaries/preview/219920
https://sistacafe.com/summaries/preview/219921
https://sistacafe.com/summaries/preview/219922
https://sistacafe.com/summaries/preview/219923
https://sistacafe.com/summaries/preview/219924
https://sistacafe.com/summaries/preview/219925
https://sistacafe.com/summaries/preview/219926
https://sistacafe.com/summaries/preview/219927
https://sistacafe.com/summaries/preview/219934
https://sistacafe.com/summaries/preview/219928
https://sistacafe.com/summaries/preview/219930
https://sistacafe.com/summaries/preview/219932
https://sistacafe.com/summaries/preview/219929
https://sistacafe.com/summaries/preview/219941
https://sistacafe.com/summaries/preview/219938
https://sistacafe.com/summaries/preview/219940
https://sistacafe.com/summaries/preview/219950
https://sistacafe.com/summaries/preview/219945
https://sistacafe.com/summaries/preview/219942
https://sistacafe.com/summaries/preview/219937
https://sistacafe.com/summaries/preview/219935
https://sistacafe.com/summaries/preview/219931
https://sistacafe.com/summaries/preview/219936
https://sistacafe.com/summaries/preview/219933
https://sistacafe.com/summaries/preview/219948
https://sistacafe.com/summaries/preview/219947
https://sistacafe.com/summaries/preview/219949
https://sistacafe.com/summaries/preview/219939
https://sistacafe.com/summaries/preview/219944
https://sistacafe.com/summaries/preview/219946
https://sistacafe.com/summaries/preview/219943
https://sistacafe.com/summaries/preview/219955
https://sistacafe.com/summaries/preview/219954
https://sistacafe.com/summaries/preview/219953
https://sistacafe.com/summaries/preview/219952
https://sistacafe.com/summaries/preview/219993
https://sistacafe.com/summaries/preview/219958
https://sistacafe.com/summaries/preview/219960
https://sistacafe.com/summaries/preview/219961
https://sistacafe.com/summaries/preview/219962
https://sistacafe.com/summaries/preview/219963
https://sistacafe.com/summaries/preview/219965
https://sistacafe.com/summaries/preview/219968
https://sistacafe.com/summaries/preview/219969
https://sistacafe.com/summaries/preview/219970
https://sistacafe.com/summaries/preview/219971
https://sistacafe.com/summaries/preview/219972
https://sistacafe.com/summaries/preview/219973
https://sistacafe.com/summaries/preview/219974
https://sistacafe.com/summaries/preview/219975
https://sistacafe.com/summaries/preview/219976
https://sistacafe.com/summaries/preview/219977
https://sistacafe.com/summaries/preview/219978
https://sistacafe.com/summaries/preview/219979
https://sistacafe.com/summaries/preview/219980
https://sistacafe.com/summaries/preview/219981
https://sistacafe.com/summaries/preview/219982
https://sistacafe.com/summaries/preview/219983
https://sistacafe.com/summaries/preview/219985
https://sistacafe.com/summaries/preview/219986
https://sistacafe.com/summaries/preview/219987
https://sistacafe.com/summaries/preview/219988
https://sistacafe.com/summaries/preview/219989
https://sistacafe.com/summaries/preview/219990
https://sistacafe.com/summaries/preview/219991
https://sistacafe.com/summaries/preview/219992
https://sistacafe.com/summaries/preview/220000
https://sistacafe.com/summaries/preview/220001
https://sistacafe.com/summaries/preview/220002
https://sistacafe.com/summaries/preview/220003
https://sistacafe.com/summaries/preview/220004
https://sistacafe.com/summaries/preview/220005
https://sistacafe.com/summaries/preview/220006
https://sistacafe.com/summaries/preview/220007
https://sistacafe.com/summaries/preview/220008
https://sistacafe.com/summaries/preview/220009
https://sistacafe.com/summaries/preview/220010
https://sistacafe.com/summaries/preview/220011
https://sistacafe.com/summaries/preview/220012
https://sistacafe.com/summaries/preview/220013
https://sistacafe.com/summaries/preview/220014
https://sistacafe.com/summaries/preview/220015
https://sistacafe.com/summaries/preview/220016
https://sistacafe.com/summaries/preview/220017
https://sistacafe.com/summaries/preview/220018
https://sistacafe.com/summaries/preview/220019
https://sistacafe.com/summaries/preview/220080
https://sistacafe.com/summaries/preview/220081
https://sistacafe.com/summaries/preview/220082
https://sistacafe.com/summaries/preview/220083
https://sistacafe.com/summaries/preview/220084
https://sistacafe.com/summaries/preview/220085
https://sistacafe.com/summaries/preview/220086
https://sistacafe.com/summaries/preview/220087
https://sistacafe.com/summaries/preview/220088
https://sistacafe.com/summaries/preview/220089
https://sistacafe.com/summaries/preview/219994
https://sistacafe.com/summaries/preview/219995
https://sistacafe.com/summaries/preview/219997
https://sistacafe.com/summaries/preview/219996
https://sistacafe.com/summaries/preview/220022
https://sistacafe.com/summaries/preview/219998
https://sistacafe.com/summaries/preview/220021
https://sistacafe.com/summaries/preview/220020
https://sistacafe.com/summaries/preview/220023
https://sistacafe.com/summaries/preview/220024
https://sistacafe.com/summaries/preview/220025
https://sistacafe.com/summaries/preview/220026
https://sistacafe.com/summaries/preview/220027
https://sistacafe.com/summaries/preview/220028
https://sistacafe.com/summaries/preview/220029
https://sistacafe.com/summaries/preview/220030
https://sistacafe.com/summaries/preview/220031
https://sistacafe.com/summaries/preview/220032
https://sistacafe.com/summaries/preview/220033
https://sistacafe.com/summaries/preview/220034
https://sistacafe.com/summaries/preview/220035
https://sistacafe.com/summaries/preview/220036
https://sistacafe.com/summaries/preview/220037
https://sistacafe.com/summaries/preview/220038
https://sistacafe.com/summaries/preview/220039
https://sistacafe.com/summaries/preview/220040
https://sistacafe.com/summaries/preview/220041
https://sistacafe.com/summaries/preview/220042
https://sistacafe.com/summaries/preview/220043
https://sistacafe.com/summaries/preview/220044
https://sistacafe.com/summaries/preview/220048
https://sistacafe.com/summaries/preview/220051
https://sistacafe.com/summaries/preview/220049
https://sistacafe.com/summaries/preview/220052
https://sistacafe.com/summaries/preview/220050
https://sistacafe.com/summaries/preview/220054
https://sistacafe.com/summaries/preview/220055
https://sistacafe.com/summaries/preview/220060
https://sistacafe.com/summaries/preview/220056
https://sistacafe.com/summaries/preview/220065
https://sistacafe.com/summaries/preview/220057
https://sistacafe.com/summaries/preview/220078
https://sistacafe.com/summaries/preview/220066
https://sistacafe.com/summaries/preview/220053
https://sistacafe.com/summaries/preview/220068
https://sistacafe.com/summaries/preview/220059
https://sistacafe.com/summaries/preview/220069
https://sistacafe.com/summaries/preview/220077
https://sistacafe.com/summaries/preview/220062
https://sistacafe.com/summaries/preview/220071
https://sistacafe.com/summaries/preview/220063
https://sistacafe.com/summaries/preview/220072
https://sistacafe.com/summaries/preview/220064
https://sistacafe.com/summaries/preview/220076
https://sistacafe.com/summaries/preview/220073
https://sistacafe.com/summaries/preview/220067
https://sistacafe.com/summaries/preview/220074
https://sistacafe.com/summaries/preview/220070
https://sistacafe.com/summaries/preview/220075
https://sistacafe.com/summaries/preview/220079
https://sistacafe.com/summaries/preview/220102
https://sistacafe.com/summaries/preview/220106
https://sistacafe.com/summaries/preview/220107
https://sistacafe.com/summaries/preview/220108
https://sistacafe.com/summaries/preview/220109
https://sistacafe.com/summaries/preview/220110
https://sistacafe.com/summaries/preview/220111
https://sistacafe.com/summaries/preview/220112
https://sistacafe.com/summaries/preview/220113
https://sistacafe.com/summaries/preview/220114
https://sistacafe.com/summaries/preview/220092
https://sistacafe.com/summaries/preview/220094
https://sistacafe.com/summaries/preview/220097
https://sistacafe.com/summaries/preview/220098
https://sistacafe.com/summaries/preview/220099
https://sistacafe.com/summaries/preview/220100
https://sistacafe.com/summaries/preview/220101
https://sistacafe.com/summaries/preview/220096
https://sistacafe.com/summaries/preview/220103
https://sistacafe.com/summaries/preview/220104
https://sistacafe.com/summaries/preview/220048
https://sistacafe.com/summaries/preview/220050
https://sistacafe.com/summaries/preview/220051
https://sistacafe.com/summaries/preview/219882
https://sistacafe.com/summaries/preview/219852
https://sistacafe.com/summaries/preview/219853
https://sistacafe.com/summaries/preview/219856
https://www.readawrite.com/a/98daef69f8e29be4e41605c2c9a62c2b
https://www.readawrite.com/a/8efddb48243391e719153a8c549d1f56
https://www.readawrite.com/a/6a1ecf276db5ae5ce023a1f46061d209
https://www.readawrite.com/a/f3ebcc921b2d723e76076155dee60c11
https://www.readawrite.com/a/4159a0f386e527b85670136912a8104f
https://www.readawrite.com/a/30972df3c8bb679f2dcc74f43445195f
https://www.readawrite.com/a/f290608acdddf792e4c29385db6b9154
https://www.readawrite.com/a/f1e3410841babfa25c3d5fe720283195
https://www.readawrite.com/a/d0497f18d2b558c6fc4541b15e81ee4a
https://www.readawrite.com/a/c8a33281a5a1ac6726d6a390d7ab5327
https://www.readawrite.com/a/57509a190a774f17b3e2d05f4e57abed
https://www.readawrite.com/a/2cfb4b82853e0ee6f2036b1c16eac0e1
https://www.readawrite.com/a/f968817c5963f1176389978fe301b027
https://www.readawrite.com/a/a55ef62192c50800b209a29b7cfd8119
https://www.readawrite.com/a/59f023855f12efceba8617bb9eabd4a9
https://www.readawrite.com/a/983769f53fbe82f2d456823f5ef0b816
https://www.readawrite.com/a/4bde3f64395c50c712af6f06ca4eff36
https://www.readawrite.com/a/ab3d0efe648b908e632da3740dea3581
https://www.readawrite.com/a/41367b34654f6f8cd51db158edf6fbc5
https://www.readawrite.com/a/3bba04c5de0c3603bb23699473070123
https://www.readawrite.com/a/cf5cd4f9f04a10b176109da4bc60b191
https://laylo.com/laylo-mcrsfpl/eXodIbh8
https://laylo.com/laylo-aveacdq/V44hucnu
https://laylo.com/laylo-b8gyoxb/f3mF6t3n
https://laylo.com/laylo-hqaljwf/IaaGjl32
https://laylo.com/laylo-agh9th0/EaQPZzAl
https://laylo.com/laylo-bohg6js/IUDbzDoM
https://laylo.com/laylo-vkoobrk/CKmAReJk
https://laylo.com/laylo-5l2wkrm/sJsrZIIe
https://laylo.com/laylo-pdtwoyi/gCnYnHye
https://laylo.com/laylo-wj2httn/fP11GFdH
https://laylo.com/laylo-dd2jlvi/pWt2oFRh
https://laylo.com/laylo-zrzmu5r/9KfK1rqb
https://laylo.com/laylo-xlhpojy/WcqKrrEk
https://laylo.com/laylo-mjua1ua/gQSmja9Y
https://laylo.com/laylo-kxdaiye/nG0BTww3
https://laylo.com/laylo-5pewzmo/fNvSVtns
https://laylo.com/laylo-fxja5b9/3GYJJmHk
https://laylo.com/laylo-gsn1jpy/3zWiUqay
https://laylo.com/laylo-txzsajm/c8aw7FEI
https://laylo.com/laylo-rwixilg/m97cocXu
https://laylo.com/laylo-s1dvbjy/z5kK1N6N
https://laylo.com/laylo-cbitamk/YfyTa4k0
https://laylo.com/laylo-ztzn6ae/Uf9EFIwT
https://laylo.com/laylo-kvbmwxa/xmS7DGkf
https://laylo.com/laylo-axxnwor/3zqwpCDC
https://laylo.com/laylo-qkkfeo1/u8jC8kgX
https://laylo.com/laylo-vdt3i2e/QDM87tQc
https://laylo.com/laylo-u3aotk5/r8Dc8C9b
https://laylo.com/laylo-n3rrfwp/8keTr148
https://laylo.com/laylo-vnxnv9k/F9XZPGCD
https://laylo.com/laylo-droxuqb/v62KHKpa
https://laylo.com/laylo-j7efaw6/rCZ2kUjZ
https://laylo.com/laylo-xmis9bn/vZ2qtsE0
https://laylo.com/laylo-pj84sqj/Hrnpo3Ni
https://laylo.com/laylo-pyynebq/lQXaofD1
https://laylo.com/laylo-allzmkv/kL9Vh1cc
https://laylo.com/laylo-allzmkv/kYsyzJdb
https://laylo.com/laylo-allzmkv/UUz4VXFO
https://laylo.com/laylo-allzmkv/Q7XRHibn
https://laylo.com/laylo-9pt7n87/eM91EHJu
https://laylo.com/laylo-9pt7n87/8S7cr4mX
https://laylo.com/laylo-9pt7n87/C5LCSTp4
https://laylo.com/laylo-9pt7n87/DdktasXz
https://laylo.com/laylo-lzczuj3/CKpN5lkB
https://laylo.com/laylo-lzczuj3/mRR7SM0L
https://laylo.com/laylo-lzczuj3/RhbOaCDm
https://laylo.com/laylo-9pt7n87/c1EfdtIK
https://laylo.com/laylo-9pt7n87/hMOWqGEf
https://laylo.com/laylo-9pt7n87/WCTHq0h4
https://laylo.com/laylo-xu4xfge/lOdBq7JL
https://laylo.com/laylo-xu4xfge/bvouDNhz
https://laylo.com/laylo-xu4xfge/Mw1I58vU
https://laylo.com/laylo-ven748x/pmz94337
https://laylo.com/laylo-ven748x/bzt2k1cU
https://laylo.com/laylo-ven748x/VbyS2Mne
https://laylo.com/laylo-ven748x/3BMqvsDD
https://laylo.com/laylo-zpthte9/mHevk9bX
https://laylo.com/laylo-zpthte9/tZcqgiw8
https://laylo.com/laylo-zpthte9/YyREQgTt
https://laylo.com/laylo-zpthte9/RWlYcrWu
https://laylo.com/laylo-iun1wgq/RBqMZ87M
https://laylo.com/laylo-iun1wgq/7oQEoHQt
https://laylo.com/laylo-iun1wgq/I99YCQ79
https://laylo.com/laylo-iun1wgq/iu344OMm
https://laylo.com/laylo-iun1wgq/WKLeqZ6n
https://laylo.com/laylo-pzuvkep/hHAoyjqb
https://laylo.com/laylo-pzuvkep/chAzia35
https://laylo.com/laylo-pzuvkep/ouyLNJsN
https://laylo.com/laylo-pzuvkep/zgJPxJbd
https://laylo.com/laylo-pzuvkep/nz5BWypb
https://laylo.com/laylo-ec9ihej/oMhYPnNT
https://laylo.com/laylo-ec9ihej/pHXeElFh
https://laylo.com/laylo-ec9ihej/El5edAjG
https://laylo.com/laylo-ec9ihej/rOZOJIVZ
https://laylo.com/laylo-ec9ihej/o0jPjCbg
https://laylo.com/laylo-azj1i8q/OMnl9aI7
https://laylo.com/laylo-azj1i8q/cQtdn7Rm
https://laylo.com/laylo-azj1i8q/BkkokSuy
https://laylo.com/laylo-azj1i8q/vSrckRg8
https://laylo.com/laylo-azj1i8q/RtLSPyS6
https://laylo.com/laylo-1lepwzt/t0Yg5tuP
https://laylo.com/laylo-ohiflqv/ITwGJPfq
https://laylo.com/laylo-hrnjg1m/vlGofcxL
https://laylo.com/laylo-qpzuuvz/ogbfs7NR
https://laylo.com/laylo-pmuwhts/VFQrBBGG
https://laylo.com/laylo-rmajsvz/nBxTrZYV
https://laylo.com/laylo-mr3ggdw/ACDshRfW
https://laylo.com/laylo-em8zo21/5Cms9YTw
https://laylo.com/laylo-dzgoqnr/J4jCCTHs
https://laylo.com/laylo-r6zrcwm/xLv3Ds5f
https://laylo.com/laylo-ekl9lwn/1BAdzkx8
https://laylo.com/laylo-hdvfhx6/7TA56Otx
https://laylo.com/laylo-qemtvpz/hCpTQJEC
https://laylo.com/laylo-ugglfiu/arO770EO
https://laylo.com/laylo-yutxaxq/f0NnOxMX
https://laylo.com/laylo-kngugmd/wMFDOxcc
https://laylo.com/laylo-yplgt5j/GBFCFTt0
https://laylo.com/laylo-irqnbfc/qkbjKriE
https://laylo.com/laylo-tzn2fxa/gchOYQ0E
https://laylo.com/laylo-lqwtzsu/MLBjfTtX
https://laylo.com/laylo-ud629kw/mP8GdUG5
https://laylo.com/laylo-rc7mk4o/jVDeesOy
https://laylo.com/laylo-vpybkax/m80JCGj3
https://laylo.com/laylo-h0xppme/tbGNzVZs
https://laylo.com/laylo-yxuw4x3/750E9loL
https://laylo.com/laylo-vlu6jce/CX5lC0m3
https://laylo.com/laylo-tjyufrn/i0ZhVhnM
https://laylo.com/laylo-5o0o7lc/QEeEgJZX
https://laylo.com/laylo-dfgbo9f/CPCuGqE8
https://laylo.com/laylo-69nrgtz/AxyQmWOo
https://laylo.com/laylo-dk1tycl/qomkYxnz
https://laylo.com/laylo-fshhjvt/B7bCHKpU
https://laylo.com/laylo-4lccwsp/agLo1oHq
https://laylo.com/laylo-dbzcwwx/fX753b9p
https://laylo.com/laylo-l4ukbky/rwUdsopR
https://laylo.com/laylo-4wqdazx/mIajbTSV
https://laylo.com/laylo-b42db9i/Mr82W2be
https://laylo.com/laylo-si7crb3/6f86Jqqh
https://laylo.com/laylo-fshhjvt/HhctXMxP
https://laylo.com/laylo-fnw7mlo/wFbWJpY7
https://laylo.com/laylo-f9hvl17/CgeLtE68
https://laylo.com/laylo-apu79ti/WCz46SJN
https://laylo.com/laylo-5xfolxo/XDyAnHiJ
https://laylo.com/laylo-mkp8k1a/rMbx3xoN
https://laylo.com/laylo-0hdch72/1kH5pwkm
https://laylo.com/laylo-uljqzmu/NYl5jfGj
https://laylo.com/laylo-3a172v2/pqctWS66
https://laylo.com/laylo-72q9osc/5JbHIqm4
https://laylo.com/laylo-rf5wunr/E1jx9hai
https://laylo.com/laylo-54gt5hu/uoGLfZs3
https://laylo.com/laylo-49ylq77/trQIcrJM
https://laylo.com/laylo-dlsog6f/r8bK0yw6
https://laylo.com/laylo-8jsfhk9/UAznnTmY
https://laylo.com/laylo-99zprcv/RxFpIHeh
https://laylo.com/laylo-rzo6bmp/SCkxbN9G
https://laylo.com/laylo-l4axgvy/VBsKMC4w
https://laylo.com/laylo-luy5fs6/Qt8fM4UW
https://laylo.com/laylo-xqt9xvj/otWs8ekw
https://laylo.com/laylo-btwb0sh/G4TetqLM
https://laylo.com/laylo-49wtrxb/obqO5oCe
https://laylo.com/laylo-oaok3s4/eSCOVwEC
https://laylo.com/laylo-bwakvtt/XupcEQ87
https://laylo.com/laylo-odgslrn/tskRqt9T
https://laylo.com/laylo-qsymosj/SLYA1sqQ
https://laylo.com/laylo-vbybkty/zw0A72wf
https://laylo.com/laylo-lt0iptd/EzYObala
https://laylo.com/laylo-wrkbq6k/XZdnra27
https://laylo.com/laylo-wtjs7wl/SDSsqwvc
https://laylo.com/laylo-x6l4vhb/IPTWJAyz
https://laylo.com/laylo-wwzxyp7/oAnHwTqd
https://laylo.com/laylo-6kiw78x/nGbRVXCS
https://laylo.com/laylo-9hsmqxh/Z1THZe9n
https://laylo.com/laylo-huhftfs/0hxboqph
https://laylo.com/laylo-1sgy04k/Tg08mA5v
https://laylo.com/laylo-lvr8rri/Jduwn0MY
https://laylo.com/laylo-gtsn2cf/bMxH1lZd
https://laylo.com/laylo-764wbw3/ISv3MgDk
https://laylo.com/laylo-8twd6t1/LYtqU79t
https://laylo.com/laylo-2gfyz3o/dAUib2mX
https://laylo.com/laylo-jbzok3a/do1riwgP
https://laylo.com/laylo-bmidket/rhY0PwCO
https://laylo.com/laylo-gadt9a9/t5NPHDwf
https://laylo.com/laylo-7axyyit/nlet0SpY
https://laylo.com/laylo-i2nqvwn/HVwIcDZt
https://laylo.com/laylo-ttaymqy/faUNsjfs
https://laylo.com/laylo-wah1brq/MTa2JZ1M
https://laylo.com/laylo-5wl0czv/oESq2xnu
https://laylo.com/laylo-den5ddc/JpoVXbs7
https://laylo.com/laylo-h9ogogp/PqNjMQLw
https://laylo.com/laylo-d6gn5ip/iHAP0MET
https://laylo.com/laylo-wcgrlxq/LnaJTv08
https://laylo.com/laylo-ywzprsw/DV1b4w5U
https://laylo.com/laylo-hjfzer3/eXeie4Ax
https://laylo.com/laylo-ljtfixo/oEdiCaoK
https://laylo.com/laylo-nauvkf6/B70imnMf
https://laylo.com/laylo-piueopc/3qLOcxVe
https://laylo.com/laylo-3aldotp/wm9XrpBG
https://laylo.com/laylo-vwz5olb/lf2B8Rk4
https://laylo.com/laylo-tn7jws5/3oHcaYr1
https://laylo.com/laylo-cpnscgn/Vy1HS9V9
https://laylo.com/laylo-hfyeaqj/peTtcdJ7
https://laylo.com/laylo-lvlnjjl/74Quc5p5
https://laylo.com/laylo-6cxttt4/gd8hLarr
https://laylo.com/laylo-u7rq55r/YjAS7nIV
https://laylo.com/laylo-vbayno4/rrYXSR0p
https://laylo.com/laylo-gnsyrrf/Q2z2LnVw
https://laylo.com/laylo-auswqju/Dovp7oUL
https://laylo.com/laylo-i92sed8/7VpEW2bf
https://laylo.com/laylo-4fjdilf/UUBUEKLs
https://laylo.com/laylo-s1np2kw/NIBeVnXQ
https://laylo.com/laylo-cpaxmbb/aJHdrVhD
https://laylo.com/laylo-flrjx7n/iJTqsZDM
https://laylo.com/laylo-wnpy8ee/BNM7MtxQ
https://laylo.com/laylo-vfzxyn5/0F4Knfcv
https://laylo.com/laylo-yf9t7xl/SJ7HYMLG
https://laylo.com/laylo-df4oskf/MJriUV7X
https://laylo.com/laylo-gjdrpgr/yfCSSweu
https://laylo.com/laylo-azkucdd/1vM34QRg
https://laylo.com/laylo-mazphwg/QnTsdpNc
https://laylo.com/laylo-2tybazd/q01ulqt6
https://laylo.com/laylo-7itph7r/nhmpzZap
https://laylo.com/laylo-7itph7r/LsWytkrE
https://laylo.com/laylo-7itph7r/OggJX8Bu
https://laylo.com/laylo-7itph7r/O1XZQbiE
https://laylo.com/laylo-7itph7r/b6eDHzUb
https://laylo.com/laylo-spbyhth/FW42Uc4y
https://laylo.com/laylo-spbyhth/JbDUhovM
https://laylo.com/laylo-spbyhth/JtrGK1en
https://laylo.com/laylo-spbyhth/yOsJpDrE
https://laylo.com/laylo-spbyhth/aYo0ERwf
https://laylo.com/laylo-9ntelby/5H1H1oFG
https://laylo.com/laylo-9ntelby/CoJ1q0uq
https://laylo.com/laylo-9ntelby/ViKtXNy3
https://laylo.com/laylo-9ntelby/g8YZEuDH
https://laylo.com/laylo-9ntelby/OfGCarPm
https://laylo.com/laylo-i9ij2oi/TcaQcTKP
https://laylo.com/laylo-i9ij2oi/AwX2ylD8
https://laylo.com/laylo-i9ij2oi/XIFrYBbD
https://laylo.com/laylo-i9ij2oi/AhG3uou4
https://laylo.com/laylo-i9ij2oi/VnkU6dWH
https://laylo.com/laylo-ufzhzcv/6sqt6kIA
https://laylo.com/laylo-ufzhzcv/QWy1bSSd
https://laylo.com/laylo-ufzhzcv/UTD5e43e
https://laylo.com/laylo-ufzhzcv/rcn6FOEo
https://laylo.com/laylo-ufzhzcv/laaYHeMt
https://laylo.com/laylo-mo0ggiy/NPTTnRu4
https://laylo.com/laylo-mo0ggiy/1JmiLkWw
https://laylo.com/laylo-0zetz06/RyuM9r3f
https://laylo.com/laylo-0zetz06/Mk0YBWRB
https://laylo.com/laylo-0zetz06/nHkIyXet
https://laylo.com/laylo-0zetz06/tQS9hoT1
https://laylo.com/laylo-0zetz06/zs3PSDiM
https://laylo.com/laylo-mo0ggiy/IVvPlEVQ
https://laylo.com/laylo-mo0ggiy/ldMPkz5J
https://laylo.com/laylo-mo0ggiy/Ghizfx7A
https://laylo.com/laylo-omps4cy/8yWhSw2h
https://laylo.com/laylo-omps4cy/MoRRQh37
https://laylo.com/laylo-omps4cy/Xe7xX7yT
https://laylo.com/laylo-omps4cy/xgZrUzbu
https://laylo.com/laylo-omps4cy/gwUWZEzV
https://laylo.com/laylo-qxbldrl/zEJKJhEK
https://laylo.com/laylo-cwmbdwu/xS6xwh39
https://laylo.com/laylo-qrryk1p/QINTMgeN
https://laylo.com/laylo-147mu4n/fMNL8wUn
https://laylo.com/laylo-db0wwgp/6JtcYVq6
https://laylo.com/laylo-wkhzwnu/VFNXO5YN
https://laylo.com/laylo-9z0i2et/8qz6cbLx
https://laylo.com/laylo-wef6zb9/kXiL8pnp
https://laylo.com/laylo-bj2ey7u/KFmVpZo9
https://laylo.com/laylo-jlfccyx/6WVqv07W
https://laylo.com/laylo-x6l4vhb/9ymbb1Iz
https://laylo.com/laylo-wwzxyp7/eYCvw13S
https://laylo.com/laylo-6kiw78x/RdpXSeLF
https://laylo.com/laylo-9hsmqxh/gcN1aRI5
https://laylo.com/laylo-huhftfs/K73nsJUf
https://laylo.com/laylo-1sgy04k/D59uxJFa
https://laylo.com/laylo-lvr8rri/F290oeo9
https://laylo.com/laylo-2gfyz3o/hZTWuNY0
https://laylo.com/laylo-gtsn2cf/acmOofZ9
https://laylo.com/laylo-764wbw3/64zsIfjf
https://laylo.com/laylo-jbzok3a/ir1U76LH
https://laylo.com/laylo-8twd6t1/5×8k3wEz
https://laylo.com/laylo-8jsfhk9/g1J28tbM
https://laylo.com/laylo-99zprcv/xJ9p1Tuk
https://laylo.com/laylo-rzo6bmp/z6urM1dP
https://laylo.com/laylo-l4axgvy/2fkP925I
https://laylo.com/laylo-luy5fs6/hghQC5CJ
https://laylo.com/laylo-xqt9xvj/Eib8HawY
https://laylo.com/laylo-btwb0sh/88zvoSBV
https://laylo.com/laylo-49wtrxb/9p8JSN2O
https://laylo.com/laylo-wtjs7wl/C9RscMUs
https://laylo.com/laylo-wrkbq6k/yimhPTms
https://laylo.com/laylo-lt0iptd/PcrZtzys
https://laylo.com/laylo-vbybkty/PAe4WOdw
https://laylo.com/laylo-qsymosj/pj6mSFBJ
https://laylo.com/laylo-odgslrn/BVbVhlmY
https://laylo.com/laylo-bwakvtt/3NUMfenO
https://laylo.com/laylo-oaok3s4/CMPgqQRg
https://laylo.com/laylo-ffxqkbe/DijMxq7l
https://laylo.com/laylo-ri4oatr/HAsQEddj
https://laylo.com/laylo-fnw7mlo/YrgyGIXF
https://laylo.com/laylo-h0xppme/PuZN4c9q
https://laylo.com/laylo-tjyufrn/fIXVEuAR
https://laylo.com/laylo-b42db9i/YBiqTuWN
https://laylo.com/laylo-vlu6jce/7LEBnAZF
https://laylo.com/laylo-yxuw4x3/jLY0TUKx
https://laylo.com/laylo-5o0o7lc/ipy5aGHz
https://laylo.com/laylo-dfgbo9f/i0KjP6De
https://laylo.com/laylo-4lccwsp/wrf5Gt2F
https://laylo.com/laylo-yutxaxq/BKqAowxq
https://laylo.com/laylo-kngugmd/jlL6R4gV
https://laylo.com/laylo-vpybkax/7fJUQLuy
https://laylo.com/laylo-ugglfiu/C29VNfEv
https://laylo.com/laylo-yplgt5j/GbizjJNC
https://laylo.com/laylo-ud629kw/elEFZiKy
https://laylo.com/laylo-irqnbfc/fpH3G8Oj
https://laylo.com/laylo-lqwtzsu/QnPNglcZ
https://laylo.com/laylo-tzn2fxa/nX9wjdAa
https://laylo.com/laylo-dk1tycl/PLdMFOj6
https://laylo.com/laylo-fshhjvt/w9R0sczH
https://laylo.com/laylo-si7crb3/bR4ivjfL
https://laylo.com/laylo-69nrgtz/ov5Rv79G
https://laylo.com/laylo-dbzcwwx/t0MRtcIv
https://laylo.com/laylo-fshhjvt/l8WwYPvF
https://laylo.com/laylo-4wqdazx/Dy1kWEIO
https://laylo.com/laylo-l4ukbky/aU8ucqlg
https://laylo.com/laylo-rc7mk4o/jVDeesOy
https://laylo.com/laylo-qemtvpz/Y85Ljyt7
https://laylo.com/laylo-ohiflqv/92y9phUr
https://laylo.com/laylo-1lepwzt/WwsPYtqH
https://laylo.com/laylo-em8zo21/kgRZCl6D
https://laylo.com/laylo-hrnjg1m/G2oNbxll
https://laylo.com/laylo-mr3ggdw/D3gyr8M9
https://laylo.com/laylo-rmajsvz/TxRx9pwq
https://laylo.com/laylo-pmuwhts/q9UtbToq
https://laylo.com/laylo-dzgoqnr/UnhMCxxN
https://laylo.com/laylo-r6zrcwm/0cXkCx7r
https://laylo.com/laylo-ekl9lwn/p2bWHXmL
https://laylo.com/laylo-hdvfhx6/r7dijHTr
https://laylo.com/laylo-qpzuuvz/60ChQfkl
https://laylo.com/laylo-7itph7r/ga2LOZKw
https://laylo.com/laylo-7itph7r/elhsNcQt
https://laylo.com/laylo-7itph7r/xxbYU4RL
https://laylo.com/laylo-7itph7r/a5br5qUc
https://laylo.com/laylo-0zetz06/Y4ACMo7M
https://laylo.com/laylo-0zetz06/3lEEHkWe
https://laylo.com/laylo-0zetz06/8fAQMjGB
https://laylo.com/laylo-0zetz06/6bzZEJt2
https://laylo.com/laylo-0zetz06/aks3E7No
https://laylo.com/laylo-i9ij2oi/WGA3KrCI
https://laylo.com/laylo-i9ij2oi/bgtspCgU
https://laylo.com/laylo-mygnzjs/IEeXQ0TV
https://laylo.com/laylo-mygnzjs/y3czX8Fh
https://laylo.com/laylo-mygnzjs/zXZTePiH
https://laylo.com/laylo-mygnzjs/YZ7esboR
https://laylo.com/laylo-mygnzjs/vNJhc3bu
https://laylo.com/laylo-omps4cy/e3Weeh70
https://laylo.com/laylo-omps4cy/K6fZs3Mv
https://laylo.com/laylo-omps4cy/JwbnfXGg
https://laylo.com/laylo-omps4cy/mhmzDM1P
https://laylo.com/laylo-omps4cy/TExiDAUj
https://laylo.com/laylo-mo0ggiy/Q9PEIhra
https://laylo.com/laylo-mo0ggiy/KYzjIqrf
https://laylo.com/laylo-mo0ggiy/xiEZdejh
https://laylo.com/laylo-mo0ggiy/Nya8HTDL
https://laylo.com/laylo-ufzhzcv/7UCEBRgs
https://laylo.com/laylo-ufzhzcv/ZBRZTKq4
https://laylo.com/laylo-ufzhzcv/GITJjZqI
https://laylo.com/laylo-spbyhth/0y0tBFFZ
https://laylo.com/laylo-spbyhth/rdrDm76C
https://laylo.com/laylo-spbyhth/VW3TT4AA
https://laylo.com/laylo-spbyhth/uVqhJVv8
https://laylo.com/laylo-spbyhth/RZFPizkH
https://laylo.com/laylo-9ntelby/QQpzY0fc
https://laylo.com/laylo-9ntelby/2JYe2BCR
https://laylo.com/laylo-9ntelby/UmgmKqud
https://laylo.com/laylo-9ntelby/tKXkVfLe
https://laylo.com/laylo-9ntelby/2oeOJjIq
https://laylo.com/laylo-spbyhth/FsrGncX9
https://laylo.com/laylo-omps4cy/2VVacwyr
https://laylo.com/laylo-ig5bpkf/6d6iGCki
https://laylo.com/laylo-gfvj8lw/FrbWANsD
https://laylo.com/laylo-fu6o3pz/W3BKGWxC
https://laylo.com/laylo-mwssx3e/EglV4gTo
https://laylo.com/laylo-x0tmyas/8wd1QXkj
https://laylo.com/laylo-o2lifhq/fmjzDzem
https://laylo.com/laylo-kjmc6yv/gv0aO6zL
https://laylo.com/laylo-fugench/vFt4th5a
https://laylo.com/laylo-3ioh0bf/swMKnkA3
https://laylo.com/laylo-cef07pq/Ro1WENJE
https://laylo.com/laylo-robxxdt/4IxqU4az
https://laylo.com/laylo-mqqejtf/hC6bAD5S
https://laylo.com/laylo-zybvlsp/gviBTHLk
https://laylo.com/laylo-kid7qef/p8gGqxJT
https://laylo.com/laylo-wfw18qe/X2fDpSDr
https://laylo.com/laylo-acaathm/HKLDPCdM
https://laylo.com/laylo-9nwzhxl/iaKEjtDd
https://laylo.com/laylo-aelusjr/x5VujiZd
https://laylo.com/laylo-ahp3wkz/g7H09gn1
https://laylo.com/laylo-vx9tr1u/XLY4qlco
https://laylo.com/laylo-cx5tjxy/WPKaMOAJ
https://laylo.com/laylo-ov6xlfs/pUlZy6xf
https://laylo.com/laylo-glqzctp/sJV9gcQ8
https://laylo.com/laylo-jzeog8p/IwG45n9X
https://laylo.com/laylo-fcybzpp/XjdWUdxt
https://laylo.com/laylo-snvtxyx/XsmKgieK
https://laylo.com/laylo-6hbjojy/E5oSq0Rx
https://laylo.com/laylo-ufb3cdi/zqJHnifn
https://laylo.com/laylo-hgsgkjl/g9QDL75A
https://laylo.com/laylo-xxuoqqq/Hk5QHMD5
https://laylo.com/laylo-bd6bq6o/CC9ZIMWG
https://laylo.com/laylo-cx1ebzf/glc5Frwi
https://laylo.com/laylo-5kxlp5j/C90tgoof
https://laylo.com/laylo-aardhhz/X2LfmUve
https://laylo.com/laylo-kifj58d/PcjSZ3O4
https://laylo.com/laylo-0i4wmow/w2mQX4qc
https://laylo.com/laylo-p8y6dsf/JyLSIdmY
https://laylo.com/laylo-yn3uwux/eTnhL3pf
https://laylo.com/laylo-ven748x/TwzBW8ph
https://laylo.com/laylo-4ceoukf/iDxActTl
https://laylo.com/laylo-tn7jws5/UBW5oEMb
https://laylo.com/laylo-azkucdd/hjoRRtLU
https://laylo.com/laylo-5wl0czv/86q1JN4t
https://laylo.com/laylo-d6gn5ip/9wGSuwBD
https://laylo.com/laylo-s1np2kw/v7KpjCgG
https://laylo.com/laylo-wcgrlxq/V8RkUjOr
https://laylo.com/laylo-i2nqvwn/UDHjaWYb
https://laylo.com/laylo-7axyyit/8gLGDWXo
https://laylo.com/laylo-piueopc/mg0rXxvB
https://laylo.com/laylo-ljtfixo/KqK9Qrxu
https://laylo.com/laylo-cpaxmbb/2W7GTxNo
https://laylo.com/laylo-ttaymqy/zaEiRrmg
https://laylo.com/laylo-vwz5olb/VLiyL8kX
https://laylo.com/laylo-6cxttt4/A5JPZL9k
https://laylo.com/laylo-i92sed8/NnuMTXLm
https://laylo.com/laylo-gnsyrrf/gLjKAG8R
https://laylo.com/laylo-cpnscgn/nNT9WShS
https://laylo.com/laylo-hfyeaqj/8NqTsMQB
https://laylo.com/laylo-df4oskf/tQfSXIK1
https://laylo.com/laylo-gjdrpgr/s9LgE9QF
https://laylo.com/laylo-4fjdilf/oaLt3A7O
https://laylo.com/laylo-yf9t7xl/8Zx1EZR0
https://laylo.com/laylo-wnpy8ee/TlvUSD9y
https://laylo.com/laylo-h9ogogp/Rxv3hITU
https://laylo.com/laylo-vfzxyn5/Vl5z4sOj
https://laylo.com/laylo-hjfzer3/IbILP7UH
https://laylo.com/laylo-den5ddc/hxRDpYeg
https://laylo.com/laylo-nauvkf6/6fXojGyv
https://laylo.com/laylo-gadt9a9/vyIkeEUe
https://laylo.com/laylo-auswqju/hbqakHJO
https://laylo.com/laylo-3aldotp/p3IxgJdl
https://laylo.com/laylo-wah1brq/70YcXnH6
https://laylo.com/laylo-u7rq55r/nqWhwkZs
https://laylo.com/laylo-2tybazd/6v6IjbjP
https://laylo.com/laylo-ywzprsw/dGmFx8Lb
https://laylo.com/laylo-vbayno4/XVIfGm1L
https://laylo.com/laylo-mazphwg/Lu2FfWXn
https://laylo.com/laylo-flrjx7n/1L7WEd52
https://laylo.com/laylo-mazphwg/Lu2FfWXn
https://laylo.com/laylo-bmidket/AmApIdZC
https://laylo.com/laylo-zgnmrho/VOuGIxBP
https://laylo.com/laylo-76bxb8z/jYr7pk4f
https://laylo.com/laylo-zgnmrho/mHdl7CSW
https://laylo.com/laylo-76bxb8z/SNWhZvCB
https://laylo.com/laylo-zgnmrho/dZ9X6i2D
https://laylo.com/laylo-76bxb8z/kCIDeKQO
https://laylo.com/laylo-zgnmrho/b6uKdIqT
https://laylo.com/laylo-76bxb8z/LRcSn2nz
https://laylo.com/laylo-zgnmrho/2MeFcjmj
https://laylo.com/laylo-76bxb8z/Fk5xHYXT
https://laylo.com/laylo-zgnmrho/lq3yj2vi
https://laylo.com/laylo-76bxb8z/rbv6zUdj
https://laylo.com/laylo-zgnmrho/od6w5OS8
https://laylo.com/laylo-76bxb8z/KAmPKcIv
https://laylo.com/laylo-76bxb8z/WgVgqyLC
https://laylo.com/laylo-zgnmrho/F7SRF36A
https://laylo.com/laylo-76bxb8z/ERx7dvp8
https://laylo.com/laylo-zgnmrho/euyM4Gfx
https://laylo.com/laylo-76bxb8z/fS7ZEug6
https://laylo.com/laylo-zgnmrho/Sxujo95D
https://laylo.com/laylo-76bxb8z/YUH83T68
https://laylo.com/laylo-6zwaqae/5teSJxPR
https://laylo.com/laylo-o4pmjna/mbFrBhYY
https://laylo.com/laylo-o4pmjna/i8c1BU0E
https://laylo.com/laylo-6zwaqae/01dPrRej
https://laylo.com/laylo-6zwaqae/0F2OW9T5
https://laylo.com/laylo-o4pmjna/98JCkOZe
https://laylo.com/laylo-o4pmjna/tAIocsS2
https://laylo.com/laylo-6zwaqae/oyfs1Gjj
https://laylo.com/laylo-o4pmjna/ZioFhpoA
https://laylo.com/laylo-6zwaqae/DlQQp2j9
https://laylo.com/laylo-o4pmjna/dGZdzgpy
https://laylo.com/laylo-6zwaqae/9mf0iHp0
https://laylo.com/laylo-o4pmjna/rJFSZ7hs
https://laylo.com/laylo-6zwaqae/Kbsgl88z
https://laylo.com/laylo-o4pmjna/YHWFuaiz
https://laylo.com/laylo-6zwaqae/HXDcsg9H
https://laylo.com/laylo-o4pmjna/PvtKSh3f
https://laylo.com/laylo-6zwaqae/CND58YXU
https://laylo.com/laylo-o4pmjna/4nzMF0Pq
https://sistacafe.com/summaries/preview/243089
https://sistacafe.com/summaries/preview/243108
https://sistacafe.com/summaries/preview/243125
https://sistacafe.com/summaries/preview/243141
https://sistacafe.com/summaries/preview/243157
https://sistacafe.com/summaries/preview/243171
https://sistacafe.com/summaries/preview/243181
https://sistacafe.com/summaries/preview/243195
https://sistacafe.com/summaries/preview/243206
https://sistacafe.com/summaries/preview/243219
https://sistacafe.com/summaries/preview/243246
https://sistacafe.com/summaries/preview/243264
https://sistacafe.com/summaries/preview/243286
https://sistacafe.com/summaries/preview/243294
https://sistacafe.com/summaries/preview/243302
https://sistacafe.com/summaries/preview/243314
https://sistacafe.com/summaries/preview/243322
https://sistacafe.com/summaries/preview/243332
https://sistacafe.com/summaries/preview/243340
https://sistacafe.com/summaries/preview/243349
https://sistacafe.com/summaries/preview/243262
https://sistacafe.com/summaries/preview/243269
https://sistacafe.com/summaries/preview/243275
https://sistacafe.com/summaries/preview/243282
https://sistacafe.com/summaries/preview/243288
https://sistacafe.com/summaries/preview/243401
https://sistacafe.com/summaries/preview/243434
https://sistacafe.com/summaries/preview/243433
https://sistacafe.com/summaries/preview/243431
https://sistacafe.com/summaries/preview/243430
https://sistacafe.com/summaries/preview/243437
https://sistacafe.com/summaries/preview/243439
https://sistacafe.com/summaries/preview/243394
https://sistacafe.com/summaries/preview/243395
https://sistacafe.com/summaries/preview/243442
https://sistacafe.com/summaries/preview/243387
https://sistacafe.com/summaries/preview/243449
https://sistacafe.com/summaries/preview/243448
https://sistacafe.com/summaries/preview/243446
https://sistacafe.com/summaries/preview/243444
https://sistacafe.com/summaries/preview/243709
https://sistacafe.com/summaries/preview/243742
https://sistacafe.com/summaries/preview/243700
https://sistacafe.com/summaries/preview/243763
https://sistacafe.com/summaries/preview/244024
https://sistacafe.com/summaries/preview/244099
https://sistacafe.com/summaries/preview/244032
https://sistacafe.com/summaries/preview/244097
https://sistacafe.com/summaries/preview/244038
https://sistacafe.com/summaries/preview/244065
https://sistacafe.com/summaries/preview/243995
https://sistacafe.com/summaries/preview/244002
https://sistacafe.com/summaries/preview/244110
https://sistacafe.com/summaries/preview/244010
https://sistacafe.com/summaries/preview/244012
https://sistacafe.com/summaries/preview/244018
https://sistacafe.com/summaries/preview/244054
https://sistacafe.com/summaries/preview/244050
https://sistacafe.com/summaries/preview/244047
https://sistacafe.com/summaries/preview/244043
https://portfolium.com/entry/top-10-4
https://portfolium.com/entry/top-10-5
https://portfolium.com/entry/top-10-6
https://portfolium.com/entry/top-10-7
https://portfolium.com/entry/top-10-8
https://portfolium.com/entry/top-10-9
https://portfolium.com/entry/top-10-10
https://portfolium.com/entry/top-10-11
https://portfolium.com/entry/top-10-12
https://portfolium.com/entry/top-10-13
https://portfolium.com/entry/24-900-1
https://portfolium.com/entry/24-900-2
https://portfolium.com/entry/24-900-3
https://portfolium.com/entry/24-900-4
https://portfolium.com/entry/24-900-5
https://portfolium.com/entry/24-900-6
https://portfolium.com/entry/24-900-7
https://portfolium.com/entry/24-900-8
https://portfolium.com/entry/24-900-9
https://portfolium.com/entry/24-900-10
https://portfolium.com/entry/tuyetngoc-bui-30129725
https://portfolium.com/entry/binhxuan-truong-30129725
https://portfolium.com/entry/minhlam-vu-30129725
https://portfolium.com/entry/thuyngoc-pho-30129725
https://portfolium.com/entry/quanghuy-ta-30129725
https://portfolium.com/entry/camchau-tieu-30129725
https://portfolium.com/entry/congnghiep-ha-30129725
https://portfolium.com/entry/huunghia-tong-30129725
https://portfolium.com/entry/duykhiem-phan-30129725
https://portfolium.com/entry/quangphong-tong-30129725
https://portfolium.com/entry/7-ab555
https://portfolium.com/entry/7-ab555-1
https://portfolium.com/entry/6-ab555
https://portfolium.com/entry/9-ab555
https://portfolium.com/entry/10-ab555
https://portfolium.com/entry/top-4-ab555
https://portfolium.com/entry/top-7-ab555
https://portfolium.com/entry/top-2-ab555
https://portfolium.com/entry/top-3-ab555
https://portfolium.com/entry/top-9-ab555
https://portfolium.com/entry/4-ab555
https://portfolium.com/entry/15-ab555
https://portfolium.com/entry/5-ab555
https://portfolium.com/entry/25-ab555
https://portfolium.com/entry/12-ab555
https://portfolium.com/entry/6-ab555-1
https://portfolium.com/entry/11-ab555
https://portfolium.com/entry/4-ab555-1
https://portfolium.com/entry/2-ab555
https://portfolium.com/entry/5-ab555-1
https://portfolium.com/entry/7-ab555-2
https://portfolium.com/entry/3-ab555
https://portfolium.com/entry/top-10-ab555-1
https://portfolium.com/entry/top-10-ab555-2
https://portfolium.com/entry/top-9-ab555-2
https://portfolium.com/entry/20-ab555
https://portfolium.com/entry/23-ab555
https://portfolium.com/entry/10-ab555-1
https://portfolium.com/entry/21-ab555
https://portfolium.com/entry/22-ab555
https://portfolium.com/entry/top-6-ab555-1
https://portfolium.com/entry/top-13-ab555
https://portfolium.com/entry/top-5-ab555
https://portfolium.com/entry/top-5-ab555-1
https://portfolium.com/entry/top-5-ab555-2
https://portfolium.com/entry/top-3-ab555-1
https://portfolium.com/entry/top-13-ab555-1
https://portfolium.com/entry/top-10-ab555
https://portfolium.com/entry/top-7-ab555-1
https://portfolium.com/entry/top-15-ab555
https://portfolium.com/entry/top-9-ab555-1
https://portfolium.com/entry/top-5-ab555-3
https://portfolium.com/entry/top-7-ab555-2
https://portfolium.com/entry/top-5-ab555-4
https://portfolium.com/entry/top-5-ab555-5
https://portfolium.com/entry/top-5-ab555-6
https://portfolium.com/entry/top-8-ab555
https://portfolium.com/entry/top-1-ab555
https://portfolium.com/entry/top-5-ab555-7
https://portfolium.com/entry/top-8-ab555-1
https://portfolium.com/entry/top-10-ab555-3
https://portfolium.com/entry/top-10-ab555-4
https://portfolium.com/entry/top-13-ab555-2
https://portfolium.com/entry/top-5-ab555-8
https://portfolium.com/entry/top-5-ab555-9
https://portfolium.com/entry/top-9-ab555-3
https://portfolium.com/entry/top-5-ab555-10
https://portfolium.com/entry/top-8-ab555-2
https://portfolium.com/entry/top-10-ab555-5
https://portfolium.com/entry/top-10-ab555-6
https://portfolium.com/entry/top-10-pg-rtp-95
https://portfolium.com/entry/top-10-pg-rtp-95-1
https://portfolium.com/entry/top-10-pg-rtp-95-2
https://portfolium.com/entry/top-10-pg-rtp-95-3
https://portfolium.com/entry/top-10-pg-rtp-95-4
https://portfolium.com/entry/top-10-pg-rtp-95-5
https://portfolium.com/entry/top-10-pg-rtp-95-6
https://portfolium.com/entry/top-10-pg-rtp-95-7
https://portfolium.com/entry/top-10-pg-rtp-95-8
https://portfolium.com/entry/top-10-pg-rtp-95-9
https://portfolium.com/entry/top-9-100-auto
https://portfolium.com/entry/top-9-100-auto-1
https://portfolium.com/entry/top-9-100-auto-2
https://portfolium.com/entry/top-9-100-auto-3
https://portfolium.com/entry/top-9-100-auto-4
https://portfolium.com/entry/top-9-100-auto-5
https://portfolium.com/entry/top-9-100-auto-6
https://portfolium.com/entry/top-9-100-auto-7
https://portfolium.com/entry/top-9-100-auto-8
https://portfolium.com/entry/top-9-100-auto-9
https://portfolium.com/entry/top-10-1-1
https://portfolium.com/entry/top-10-1-2
https://portfolium.com/entry/top-10-1-3
https://portfolium.com/entry/top-10-1-4
https://portfolium.com/entry/top-10-1-5
https://portfolium.com/entry/top-10-1-6
https://portfolium.com/entry/top-10-1-7
https://portfolium.com/entry/top-10-1-8
https://portfolium.com/entry/top-10-1-9
https://portfolium.com/entry/top-10-1-10
https://portfolium.com/entry/top-10-1-11
https://portfolium.com/entry/top-10-1-12
https://portfolium.com/entry/top-10-1-13
https://portfolium.com/entry/top-10-1-14
https://portfolium.com/entry/top-10-1-15
https://portfolium.com/entry/7-api
https://portfolium.com/entry/7-pg-1
https://portfolium.com/entry/6-pg-1
https://portfolium.com/entry/8-pg-1
https://portfolium.com/entry/9-pg-1
https://portfolium.com/entry/5-pg-1
https://portfolium.com/entry/11-pg-1
https://portfolium.com/entry/10-pg-1
https://portfolium.com/entry/4-pg-1
https://portfolium.com/entry/12-pg-1
https://portfolium.com/entry/13-pg-1
https://portfolium.com/entry/14-pg-1
https://portfolium.com/entry/9-1
https://portfolium.com/entry/10-1
https://portfolium.com/entry/8-1
https://portfolium.com/entry/4-1
https://portfolium.com/entry/6-1
https://portfolium.com/entry/7-1
https://portfolium.com/entry/top-10-1-ab555
https://portfolium.com/entry/14-1
https://portfolium.com/entry/11-1
https://portfolium.com/entry/5-1
https://portfolium.com/entry/top-7-1-ab555
https://portfolium.com/entry/top-4-1-ab555
https://portfolium.com/entry/top-6-1-ab555
https://portfolium.com/entry/top-5-1-ab555
https://portfolium.com/entry/top-3-1-ab555
https://portfolium.com/entry/top-2-1-ab555
https://portfolium.com/entry/10-1-ab555
https://portfolium.com/entry/20-1-ab555
https://portfolium.com/entry/top-1-1-ab555
https://portfolium.com/entry/top-9-1-ab555
https://portfolium.com/entry/top-5-1-ab555-1
https://portfolium.com/entry/14-1-ab555
https://portfolium.com/entry/top-7-1-ab555-1
https://portfolium.com/entry/top-7-api
https://portfolium.com/entry/top-9-api
https://portfolium.com/entry/top-8-api
https://portfolium.com/entry/top-5-api
https://portfolium.com/entry/top-6-api
https://portfolium.com/entry/top-2-api
https://portfolium.com/entry/top-4-api
https://portfolium.com/entry/top-1-api
https://portfolium.com/entry/top-10-api
https://portfolium.com/entry/top-12-api
https://portfolium.com/entry/top-13-api
https://portfolium.com/entry/top-7-api-1
https://portfolium.com/entry/top-7-api-2
https://portfolium.com/entry/top-16-api
https://portfolium.com/entry/top-10-api-1
https://portfolium.com/entry/top-7-api-3
https://portfolium.com/entry/top-7-api-4
https://portfolium.com/entry/top-10-api-2
https://portfolium.com/entry/top-8-api-1
https://portfolium.com/entry/top-12-api-1
https://portfolium.com/entry/top-9-api-1
https://portfolium.com/entry/top-10-api-3
https://portfolium.com/entry/top-4-api-1
https://portfolium.com/entry/top-3-api
https://portfolium.com/entry/top-5-api-1
https://portfolium.com/entry/top-13-api-1
https://portfolium.com/entry/top-14-api
https://portfolium.com/entry/top-17-api
https://portfolium.com/entry/top-15-api
https://portfolium.com/entry/top-12-api-2
https://portfolium.com/entry/top-13-api-2
https://portfolium.com/entry/top-7-api-5
https://portfolium.com/entry/top-7-api-6
https://portfolium.com/entry/top-7-api-7
https://portfolium.com/entry/top-7-api-8
https://portfolium.com/entry/top-10-1-16
https://portfolium.com/entry/top-10-1-17
https://portfolium.com/entry/top-10-1-18
https://portfolium.com/entry/top-10-1-19
https://portfolium.com/entry/top-10-1-20
https://portfolium.com/entry/top-10-1-21
https://portfolium.com/entry/top-10-1-22
https://portfolium.com/entry/top-10-1-23
https://portfolium.com/entry/top-10-1-24
https://portfolium.com/entry/top-10-1-25
https://portfolium.com/entry/top-7-1
https://portfolium.com/entry/top-7-1-1
https://portfolium.com/entry/top-7-1-2
https://portfolium.com/entry/top-7-1-3
https://portfolium.com/entry/top-7-1-4
https://portfolium.com/entry/top-7-1-5
https://portfolium.com/entry/top-7-1-6
https://portfolium.com/entry/top-7-1-7
https://portfolium.com/entry/top-7-1-8
https://portfolium.com/entry/top-7-1-9
https://portfolium.com/entry/top-10-14
https://portfolium.com/entry/top-10-15
https://portfolium.com/entry/top-10-16
https://portfolium.com/entry/top-10-17
https://portfolium.com/entry/top-10-18
https://portfolium.com/entry/top-10-19
https://portfolium.com/entry/top-10-20
https://portfolium.com/entry/top-10-21
https://portfolium.com/entry/top-10-22
https://portfolium.com/entry/11-13
https://portfolium.com/entry/sorelzdarna93-phae-13
https://portfolium.com/entry/10-2028
https://portfolium.com/entry/solariadarna05-phae-12
https://portfolium.com/entry/veeraphong-phae-14
https://portfolium.com/entry/boomkity-phae-15
https://portfolium.com/entry/inorce-darna-phae-16
https://portfolium.com/entry/4-92074
https://portfolium.com/entry/arofiah-phae-7
https://portfolium.com/entry/6-2036
https://portfolium.com/entry/afzal-khan-8
https://portfolium.com/entry/afzal-khan-7
https://portfolium.com/entry/5-2051
https://portfolium.com/entry/5-2052
https://portfolium.com/entry/6-2037
https://portfolium.com/entry/afzal-khan-8-1
https://portfolium.com/entry/9-92034
https://portfolium.com/entry/6-2038
https://portfolium.com/entry/10-2029
https://portfolium.com/entry/4-92075
https://portfolium.com/entry/ali-jan-22
https://portfolium.com/entry/10-2030
https://portfolium.com/entry/wllm-rbrt-12
https://portfolium.com/entry/11-14
https://portfolium.com/entry/afzal-khan-13
https://portfolium.com/entry/afzal-khan-14
https://portfolium.com/entry/afzal-khan-20
https://portfolium.com/entry/rofi-phae-15
https://portfolium.com/entry/frinkl-dyt-16
https://portfolium.com/entry/kaisya-phae-23
https://portfolium.com/entry/10-2027
https://portfolium.com/entry/10-2031
https://portfolium.com/entry/10-2032
https://portfolium.com/entry/10-2033
https://portfolium.com/entry/wily-darna-12
https://portfolium.com/entry/wily-darna-13
https://portfolium.com/entry/ikram-darna-8
https://portfolium.com/entry/ikram-darna-7
https://portfolium.com/entry/6-2039
https://portfolium.com/entry/5-2053
https://portfolium.com/entry/4-92076
https://portfolium.com/entry/10-2038
https://portfolium.com/entry/10-2037
https://portfolium.com/entry/10-2036
https://portfolium.com/entry/10-2035
https://portfolium.com/entry/10-2034
https://portfolium.com/entry/iqbal-darna-8-1
https://portfolium.com/entry/iqbal-darna-7-1
https://portfolium.com/entry/9-92035
https://portfolium.com/entry/iqbal-darna-8
https://portfolium.com/entry/iqbal-darna-7
https://portfolium.com/entry/1-258
https://portfolium.com/entry/melea-darna-2
https://portfolium.com/entry/melea-darna-3
https://portfolium.com/entry/4-92077
https://portfolium.com/entry/5-2054
https://portfolium.com/entry/yudha-darna-13
https://portfolium.com/entry/yudha-darna-12
https://portfolium.com/entry/11-15
https://portfolium.com/entry/10-2040
https://portfolium.com/entry/10-2039
https://portfolium.com/entry/10-2041
https://portfolium.com/entry/10-2042
https://portfolium.com/entry/6-2040
https://portfolium.com/entry/6-2041
https://portfolium.com/entry/9-92036
https://portfolium.com/entry/9-92037
https://portfolium.com/entry/6-2042
https://portfolium.com/entry/5-2055
https://portfolium.com/entry/felixdarna44-free-8
https://portfolium.com/entry/oktadarna12-free-7
https://portfolium.com/entry/top10
https://portfolium.com/entry/top10-1
https://portfolium.com/entry/top10-2
https://portfolium.com/entry/top10-3
https://portfolium.com/entry/top10-4
https://portfolium.com/entry/top10-5
https://portfolium.com/entry/top10-6
https://portfolium.com/entry/top10-7
https://portfolium.com/entry/top10-8
https://portfolium.com/entry/top10-9
https://portfolium.com/entry/10-2043
https://portfolium.com/entry/10-2044
https://portfolium.com/entry/10-2045
https://portfolium.com/entry/10-2046
https://portfolium.com/entry/10-2047
https://portfolium.com/entry/10-2048
https://portfolium.com/entry/10-2049
https://portfolium.com/entry/10-2050
https://portfolium.com/entry/10-2051
https://portfolium.com/entry/10-2052
https://portfolium.com/entry/top-7-100
https://portfolium.com/entry/top-7-100-1
https://portfolium.com/entry/top-7-100-2
https://portfolium.com/entry/top-7-100-3
https://portfolium.com/entry/top-7-100-4
https://portfolium.com/entry/top-7-100-5
https://portfolium.com/entry/top-7-100-6
https://portfolium.com/entry/top-7-100-7
https://portfolium.com/entry/top-7-100-8
https://portfolium.com/entry/top-7-100-9
https://portfolium.com/entry/top-5-lineab555
https://portfolium.com/entry/top-9-lineab555
https://portfolium.com/entry/top-8-lineab555
https://portfolium.com/entry/top-7-lineab555
https://portfolium.com/entry/top-6-lineab555
https://portfolium.com/entry/top-1-lineab555
https://portfolium.com/entry/top-3-lineab555
https://portfolium.com/entry/top-4-lineab555
https://portfolium.com/entry/top-10-ab555-8
https://portfolium.com/entry/top-2-lineab555
https://portfolium.com/entry/top-5-lineab555-1
https://portfolium.com/entry/top-6-lineab555-2
https://portfolium.com/entry/top-7-lineab555-1
https://portfolium.com/entry/top-6-lineab555-1
https://portfolium.com/entry/top-8-lineab555-1
https://portfolium.com/entry/top-7-lineab555-2
https://portfolium.com/entry/top-5-lineab555-2
https://portfolium.com/entry/top-5-lineab555-3
https://portfolium.com/entry/top-1-lineab555-1
https://portfolium.com/entry/top-2-lineab555-1
https://portfolium.com/entry/top-13-ab555-3
https://portfolium.com/entry/top-12-ab555
https://portfolium.com/entry/top-10-ab555-9
https://portfolium.com/entry/top-11-ab555
https://portfolium.com/entry/top-13-ab555-4
https://portfolium.com/entry/8-ab555
https://portfolium.com/entry/10-ab555-5
https://portfolium.com/entry/7-ab555-3
https://portfolium.com/entry/6-ab555-2
https://portfolium.com/entry/5-ab555-2
https://portfolium.com/entry/13-ab555
https://portfolium.com/entry/14-ab555
https://portfolium.com/entry/16-ab555
https://portfolium.com/entry/15-ab555-1
https://portfolium.com/entry/10-ab555-2
https://portfolium.com/entry/11-ab555-1
https://portfolium.com/entry/9-ab555-1
https://portfolium.com/entry/12-ab555-1
https://portfolium.com/entry/17-ab555
https://portfolium.com/entry/top-10-ab555-7
https://portfolium.com/entry/top-4-ab555-2
https://portfolium.com/entry/top-9-ab555-4
https://portfolium.com/entry/top-8-ab555-3
https://portfolium.com/entry/top-7-ab555-3
https://portfolium.com/entry/top-2-ab555-1
https://portfolium.com/entry/top-6-ab555-2
https://portfolium.com/entry/top-3-ab555-2
https://portfolium.com/entry/top-5-ab555-11
https://portfolium.com/entry/14-ab555-1
https://portfolium.com/entry/15-ab555-2
https://portfolium.com/entry/20-ab555-1
https://portfolium.com/entry/21-ab555-1
https://portfolium.com/entry/14-ab555-2
https://portfolium.com/entry/10-ab555-3
https://portfolium.com/entry/19-ab555
https://portfolium.com/entry/24-ab555
https://portfolium.com/entry/22-ab555-1
https://portfolium.com/entry/12-ab555-2
https://portfolium.com/entry/23-ab555-1
https://portfolium.com/entry/26-ab555
https://portfolium.com/entry/14-ab555-3
https://portfolium.com/entry/10-ab555-4
https://portfolium.com/entry/4-ab555-2
https://portfolium.com/entry/9-ab555-2
https://portfolium.com/entry/14-ab555-4
https://portfolium.com/entry/top-10-101
https://portfolium.com/entry/top-10-102
https://portfolium.com/entry/top-10-103
https://portfolium.com/entry/top-10-104
https://portfolium.com/entry/top-10-105
https://portfolium.com/entry/top-10-106
https://portfolium.com/entry/top-10-107
https://portfolium.com/entry/top-10-108
https://portfolium.com/entry/top-10-109
https://portfolium.com/entry/top-10-110
https://portfolium.com/entry/thtop-7
https://portfolium.com/entry/thtop-7-1
https://portfolium.com/entry/thtop-7-2
https://portfolium.com/entry/thtop-7-3
https://portfolium.com/entry/thtop-7-4
https://portfolium.com/entry/thtop-7-5
https://portfolium.com/entry/thtop-7-6
https://portfolium.com/entry/thtop-7-7
https://portfolium.com/entry/thtop-7-8
https://portfolium.com/entry/thtop-7-9
https://portfolium.com/entry/10-2053
https://portfolium.com/entry/10-2054
https://portfolium.com/entry/10-2055
https://portfolium.com/entry/10-2056
https://portfolium.com/entry/10-2057
https://portfolium.com/entry/10-2058
https://portfolium.com/entry/10-2059
https://portfolium.com/entry/10-2060
https://portfolium.com/entry/10-2061
https://portfolium.com/entry/10-2062
https://portfolium.com/entry/top-9-1
https://portfolium.com/entry/top-9-2
https://portfolium.com/entry/top-9-3
https://portfolium.com/entry/top-9-4
https://portfolium.com/entry/top-9-5
https://portfolium.com/entry/top-10-100th
https://portfolium.com/entry/14-ab555-1-1
https://portfolium.com/entry/13-ab555-1
https://portfolium.com/entry/11-ab555-1-1
https://portfolium.com/entry/12-ab555-1-1
https://portfolium.com/entry/top-9-ab555-5
https://portfolium.com/entry/top-8-100th
https://portfolium.com/entry/top-9-100th
https://portfolium.com/entry/top-7-100th
https://portfolium.com/entry/top-6-100th
https://portfolium.com/entry/6-ab555-1-1
https://portfolium.com/entry/7-ab555-1-1
https://portfolium.com/entry/15-ab555-1-1
https://portfolium.com/entry/16-ab555-1
https://portfolium.com/entry/17-ab555-1
https://portfolium.com/entry/4-ab555-1-1
https://portfolium.com/entry/5-ab555-1-1
https://portfolium.com/entry/9-ab555-1-1
https://portfolium.com/entry/23-ab555-1-1
https://portfolium.com/entry/24-ab555-1
https://portfolium.com/entry/17-100
https://portfolium.com/entry/10-100
https://portfolium.com/entry/11-100
https://portfolium.com/entry/12-100
https://portfolium.com/entry/13-100
https://portfolium.com/entry/6-100
https://portfolium.com/entry/7-100
https://portfolium.com/entry/4-100
https://portfolium.com/entry/9-100
https://portfolium.com/entry/10-100-1
https://portfolium.com/entry/4-100th
https://portfolium.com/entry/6-100th
https://portfolium.com/entry/7-100th
https://portfolium.com/entry/8-100th
https://portfolium.com/entry/9-100th
https://portfolium.com/entry/10-ab555-1-1
https://portfolium.com/entry/5-100
https://portfolium.com/entry/10-100-2
https://portfolium.com/entry/12-100-1
https://portfolium.com/entry/11-100-1
https://portfolium.com/entry/top-11-ab555-1
https://portfolium.com/entry/top-2-ab555-1-1
https://portfolium.com/entry/top-3-ab555-1-1
https://portfolium.com/entry/top-4-ab555-1-1
https://portfolium.com/entry/top-9-ab555-1-1
https://portfolium.com/entry/top-10-ab555-10
https://portfolium.com/entry/top-8-ab555-4
https://portfolium.com/entry/top-7-ab555-4
https://portfolium.com/entry/top-6-ab555-3
https://portfolium.com/entry/top-5-ab555-12
https://portfolium.com/entry/top-10-ab555-11
https://portfolium.com/entry/top-2-ab555-2
https://portfolium.com/entry/top-3-ab555-3
https://portfolium.com/entry/top-4-ab555-3
https://portfolium.com/entry/top-10-ab555-12
https://portfolium.com/entry/top-10-ab555-13
https://portfolium.com/entry/top-9-ab555-6
https://portfolium.com/entry/top-8-ab555-5
https://portfolium.com/entry/top-7-ab555-5
https://portfolium.com/entry/top-6-ab555-4
https://portfolium.com/entry/top-7-lineab555-2
https://portfolium.com/entry/top-5-lineab555-2
https://portfolium.com/entry/top-5-lineab555-3
https://portfolium.com/entry/top-1-lineab555-1
https://portfolium.com/entry/top-2-lineab555-1
https://portfolium.com/entry/top-13-ab555-3
https://portfolium.com/entry/top-12-ab555
https://portfolium.com/entry/top-10-ab555-9
https://portfolium.com/entry/top-11-ab555
https://portfolium.com/entry/top-13-ab555-4
https://portfolium.com/entry/nasi-goreng-tarka-30129725
https://portfolium.com/entry/kedai-tarka-30129725
https://portfolium.com/entry/saus-tomat-tarka-30129725
https://portfolium.com/entry/kipas-tarka-30129725
https://portfolium.com/entry/rumah-sakit-tarka-30129725
https://portfolium.com/entry/keripik-singkong-tarka-30129725
https://portfolium.com/entry/athaya-free-30129725
https://portfolium.com/entry/aditarka-10-30129725
https://portfolium.com/entry/ujitarka-11-30129725
https://portfolium.com/entry/uditarka-12-30129725
https://portfolium.com/entry/top10-10
https://portfolium.com/entry/top10-11
https://portfolium.com/entry/top10-12
https://portfolium.com/entry/top10-13
https://portfolium.com/entry/top10-14
https://portfolium.com/entry/top10-15
https://portfolium.com/entry/top10-16
https://portfolium.com/entry/top10-17
https://portfolium.com/entry/top10-18
https://portfolium.com/entry/top10-19
https://portfolium.com/entry/6-2043
https://portfolium.com/entry/6-2044
https://portfolium.com/entry/6-2045
https://portfolium.com/entry/6-2046
https://portfolium.com/entry/6-2047
https://portfolium.com/entry/6-2048
https://portfolium.com/entry/6-2049
https://portfolium.com/entry/6-2050
https://portfolium.com/entry/6-2051
https://portfolium.com/entry/6-2052
https://portfolium.com/entry/24-900-11
https://portfolium.com/entry/24-900-12
https://portfolium.com/entry/24-900-13
https://portfolium.com/entry/24-900-14
https://portfolium.com/entry/24-900-15
https://portfolium.com/entry/6-ab555-1-2
https://portfolium.com/entry/13-ab555-1-1
https://portfolium.com/entry/12-ab555-1-2
https://portfolium.com/entry/10-ab555-1-2
https://portfolium.com/entry/16-ab555-1-1
https://portfolium.com/entry/6-ab555-1-3
https://portfolium.com/entry/7-ab555-1-2
https://portfolium.com/entry/8-ab555-1-1
https://portfolium.com/entry/9-ab555-1-2
https://portfolium.com/entry/4-ab555-1-2
https://portfolium.com/entry/18-ab555-1
https://portfolium.com/entry/17-ab555-1-1
https://portfolium.com/entry/15-ab555-1-2
https://portfolium.com/entry/14-ab555-1-2
https://portfolium.com/entry/10-ab555-1-3
https://portfolium.com/entry/6-ab555-1-4
https://portfolium.com/entry/9-ab555-1-3
https://portfolium.com/entry/1-ab555-1
https://portfolium.com/entry/2-ab555-1-1
https://portfolium.com/entry/11-ab555-1-2
https://portfolium.com/entry/6-ab555-1-5
https://portfolium.com/entry/7-ab555-1-3
https://portfolium.com/entry/10-ab555-1-4
https://portfolium.com/entry/6-ab555-1-6
https://portfolium.com/entry/6-ab555-1-7
https://portfolium.com/entry/4-ab555-1-3
https://portfolium.com/entry/5-ab555-1-2
https://portfolium.com/entry/3-ab555-1-1
https://portfolium.com/entry/15-ab555-1-3
https://portfolium.com/entry/16-ab555-1-2
https://portfolium.com/entry/17-ab555-1-2
https://portfolium.com/entry/14-ab555-1-3
https://portfolium.com/entry/13-ab555-1-2
https://portfolium.com/entry/12-ab555-1-3
https://portfolium.com/entry/10-ab555-1-5
https://portfolium.com/entry/8-ab555-1
https://portfolium.com/entry/9-ab555-3
https://portfolium.com/entry/11-ab555-2
https://portfolium.com/entry/10-ab555-6
https://portfolium.com/entry/12-ab555-3
https://portfolium.com/entry/13-ab555-2
https://portfolium.com/entry/3-ab555-1
https://portfolium.com/entry/2-ab555-1
https://portfolium.com/entry/5-ab555-3
https://portfolium.com/entry/4-ab555-3
https://portfolium.com/entry/7-ab555-4
https://portfolium.com/entry/6-ab555-3
https://portfolium.com/entry/14-ab555-5
https://portfolium.com/entry/15-ab555-3
https://portfolium.com/entry/16-ab555-2
https://portfolium.com/entry/17-ab555-2
https://portfolium.com/entry/19-ab555-1
https://portfolium.com/entry/18-ab555
https://portfolium.com/entry/top-10-ab555-14
https://portfolium.com/entry/top-1-ab555-1
https://portfolium.com/entry/top-3-ab555-4
https://portfolium.com/entry/top-2-ab555-3
https://portfolium.com/entry/top-8-ab555-6
https://portfolium.com/entry/top-9-ab555-9
https://portfolium.com/entry/top-1-ab555-2
https://portfolium.com/entry/top-8-ab555-7
https://portfolium.com/entry/top-7-ab555-6
https://portfolium.com/entry/top-10-ab555-15
https://portfolium.com/entry/top-5-ab555-13
https://portfolium.com/entry/10-ab555-7
https://portfolium.com/entry/7-ab555-5
https://portfolium.com/entry/4-ab555-4
https://portfolium.com/entry/8-ab555-2
https://portfolium.com/entry/9-ab555-4
https://portfolium.com/entry/8-ab555-1-2
https://portfolium.com/entry/10-100-3
https://portfolium.com/entry/9-100-1
https://portfolium.com/entry/8-100
https://portfolium.com/entry/7-100-1
https://portfolium.com/entry/6-100-1
https://portfolium.com/entry/10-api-100
https://portfolium.com/entry/10-api-100-1
https://portfolium.com/entry/10-api-100-2
https://portfolium.com/entry/10-api-100-3
https://portfolium.com/entry/10-api-100-4
https://portfolium.com/entry/10-api-100-5
https://portfolium.com/entry/10-api-100-6
https://portfolium.com/entry/10-api-100-7
https://portfolium.com/entry/10-api-100-8
https://portfolium.com/entry/10-api-100-9
https://portfolium.com/entry/pg-rtp
https://portfolium.com/entry/pg-rtp-1
https://portfolium.com/entry/pg-rtp-2
https://portfolium.com/entry/pg-rtp-3
https://portfolium.com/entry/pg-rtp-4
https://portfolium.com/entry/pg-rtp-5
https://portfolium.com/entry/pg-rtp-6
https://portfolium.com/entry/pg-rtp-7
https://portfolium.com/entry/pg-rtp-8
https://portfolium.com/entry/pg-rtp-9
https://portfolium.com/entry/slot-pg-auto
https://portfolium.com/entry/slot-pg-auto-1
https://portfolium.com/entry/slot-pg-auto-2
https://portfolium.com/entry/slot-pg-auto-3
https://portfolium.com/entry/slot-pg-auto-4
https://portfolium.com/entry/slot-pg-auto-5
https://portfolium.com/entry/slot-pg-auto-6
https://portfolium.com/entry/slot-pg-auto-7
https://portfolium.com/entry/slot-pg-auto-8
https://portfolium.com/entry/slot-pg-auto-9
https://portfolium.com/entry/6-ab555-1-2
https://portfolium.com/entry/13-ab555-1-1
https://portfolium.com/entry/12-ab555-1-2
https://portfolium.com/entry/10-ab555-1-2
https://portfolium.com/entry/16-ab555-1-1
https://portfolium.com/entry/6-ab555-1-3
https://portfolium.com/entry/7-ab555-1-2
https://portfolium.com/entry/8-ab555-1-1
https://portfolium.com/entry/9-ab555-1-2
https://portfolium.com/entry/4-ab555-1-2
https://portfolium.com/entry/18-ab555-1
https://portfolium.com/entry/17-ab555-1-1
https://portfolium.com/entry/15-ab555-1-2
https://portfolium.com/entry/14-ab555-1-2
https://portfolium.com/entry/10-ab555-1-3
https://portfolium.com/entry/6-ab555-1-4
https://portfolium.com/entry/9-ab555-1-3
https://portfolium.com/entry/1-ab555-1
https://portfolium.com/entry/2-ab555-1-1
https://portfolium.com/entry/11-ab555-1-2
https://portfolium.com/entry/6-ab555-1-5
https://portfolium.com/entry/7-ab555-1-3
https://portfolium.com/entry/10-ab555-1-4
https://portfolium.com/entry/6-ab555-1-6
https://portfolium.com/entry/6-ab555-1-7
https://portfolium.com/entry/4-ab555-1-3
https://portfolium.com/entry/5-ab555-1-2
https://portfolium.com/entry/3-ab555-1-1
https://portfolium.com/entry/15-ab555-1-3
https://portfolium.com/entry/16-ab555-1-2
https://portfolium.com/entry/17-ab555-1-2
https://portfolium.com/entry/14-ab555-1-3
https://portfolium.com/entry/13-ab555-1-2
https://portfolium.com/entry/12-ab555-1-3
https://portfolium.com/entry/10-ab555-1-5
https://portfolium.com/entry/top-4-ab555-4
https://portfolium.com/entry/top-5-ab555-14
https://portfolium.com/entry/top-7-ab555-7
https://portfolium.com/entry/top-8-ab555-8
https://portfolium.com/entry/top-6-ab555-6
https://portfolium.com/entry/top-9-ab555-10
https://portfolium.com/entry/9-ab555-5
https://portfolium.com/entry/4-ab555-5
https://portfolium.com/entry/5-ab555-4
https://portfolium.com/entry/6-ab555-4
https://portfolium.com/entry/8-ab555-3
https://portfolium.com/entry/7-ab555-6
https://portfolium.com/entry/13-pg-1-1
https://portfolium.com/entry/7-pg-1-1
https://portfolium.com/entry/10-pg-1-1
https://portfolium.com/entry/11-pg-1-1
https://portfolium.com/entry/16-pg-1
https://portfolium.com/entry/17-pg-1
https://portfolium.com/entry/10-pg-1-2
https://portfolium.com/entry/7-pg-1-2
https://portfolium.com/entry/18-pg-1
https://portfolium.com/entry/9-pg-1-1
https://portfolium.com/entry/27-pg-1
https://portfolium.com/entry/6-pg-1-1
https://portfolium.com/entry/13-pg-1-2
https://portfolium.com/entry/19-pg-1
https://portfolium.com/entry/12-pg-1-1
https://portfolium.com/entry/23-pg-1
https://portfolium.com/entry/5-pg-1-1
https://portfolium.com/entry/22-pg-1
https://portfolium.com/entry/14-pg-1-1
https://portfolium.com/entry/15-pg-1
https://portfolium.com/entry/21-pg-1
https://portfolium.com/entry/20-pg-1
https://portfolium.com/entry/top-8-1
https://portfolium.com/entry/top-4-1
https://portfolium.com/entry/top-5-1-1
https://portfolium.com/entry/top-6-1
https://portfolium.com/entry/top-7-1-10
https://portfolium.com/entry/top-7-2569
https://portfolium.com/entry/top-7-2570
https://portfolium.com/entry/top-7-2571
https://portfolium.com/entry/top-7-2572
https://portfolium.com/entry/top-7-2573
https://portfolium.com/entry/top-7-2574
https://portfolium.com/entry/top-7-2575
https://portfolium.com/entry/top-7-2576
https://portfolium.com/entry/top-7-2577
https://portfolium.com/entry/top-7-2578
https://portfolium.com/entry/top-10-111
https://portfolium.com/entry/top-10-112
https://portfolium.com/entry/top-10-113
https://portfolium.com/entry/top-10-114
https://portfolium.com/entry/top-10-115
https://portfolium.com/entry/top-10-116
https://portfolium.com/entry/top-10-117
https://portfolium.com/entry/top-10-118
https://portfolium.com/entry/top-10-119
https://portfolium.com/entry/top-10-120
https://portfolium.com/entry/top-9-100
https://portfolium.com/entry/top-9-100-1
https://portfolium.com/entry/top-9-100-2
https://portfolium.com/entry/top-9-100-3
https://portfolium.com/entry/top-9-100-4
https://portfolium.com/entry/top-9-100-5
https://portfolium.com/entry/top-9-100-6
https://portfolium.com/entry/top-9-100-7
https://portfolium.com/entry/top-9-100-8
https://portfolium.com/entry/top-9-100-9
https://portfolium.com/entry/4-92078
https://portfolium.com/entry/5-2056
https://portfolium.com/entry/rahmatarka-phae-2
https://portfolium.com/entry/rahmatarka-phae-3
https://portfolium.com/entry/6-2053
https://portfolium.com/entry/pabuarantarka-phae-7
https://portfolium.com/entry/fiendratarka-phae-8
https://portfolium.com/entry/9-92038
https://portfolium.com/entry/10-2063
https://portfolium.com/entry/9-92039
https://portfolium.com/entry/apudtarka-phae-7
https://portfolium.com/entry/apudtarka-phae-8
https://portfolium.com/entry/6-2054
https://portfolium.com/entry/5-2057
https://portfolium.com/entry/10-2064
https://portfolium.com/entry/11-101
https://portfolium.com/entry/11-102
https://portfolium.com/entry/10-2065
https://portfolium.com/entry/guguntarka-phae-12
https://portfolium.com/entry/guguntarka-phae-13
https://portfolium.com/entry/ezzartarka-phae-20
https://portfolium.com/entry/ezzartarka-phae-21
https://portfolium.com/entry/adnan-khadim-15
https://portfolium.com/entry/adnan-khadim-14
https://portfolium.com/entry/teditarka-phae-16
https://portfolium.com/entry/teditarka-phae-17
https://portfolium.com/entry/teditarka-phae-16-1
https://portfolium.com/entry/11-103
https://portfolium.com/entry/editarka-phae-12
https://portfolium.com/entry/ezzartarka-phae-17
https://portfolium.com/entry/guguntarka-phae-14
https://portfolium.com/entry/10-2066
https://portfolium.com/entry/apudtarka-phae-15
https://portfolium.com/entry/ulungtarka-phae-13
https://portfolium.com/entry/10-2067
https://portfolium.com/entry/top-1
https://portfolium.com/entry/top-10-121
https://portfolium.com/entry/top-11-1
https://portfolium.com/entry/top-12
https://portfolium.com/entry/top-13
https://portfolium.com/entry/top-18
https://portfolium.com/entry/top-17
https://portfolium.com/entry/top-15
https://portfolium.com/entry/top-14
https://portfolium.com/entry/top-6
https://portfolium.com/entry/top-7-2579
https://portfolium.com/entry/top-8
https://portfolium.com/entry/top-9-101
https://portfolium.com/entry/top-10-122
https://portfolium.com/entry/6-2055
https://portfolium.com/entry/rijaldarna35-htfh-7
https://portfolium.com/entry/rijaldarna35-htfh-8
https://portfolium.com/entry/9-92040
https://portfolium.com/entry/10-2068
https://portfolium.com/entry/4-92079
https://portfolium.com/entry/rafi-da-2
https://portfolium.com/entry/1-273
https://portfolium.com/entry/rafi-da-3
https://portfolium.com/entry/5-2058
https://portfolium.com/entry/padil-kalam-16
https://portfolium.com/entry/padil-kalam-17
https://portfolium.com/entry/padil-kalam-18
https://portfolium.com/entry/10-2069
https://portfolium.com/entry/11-104
https://portfolium.com/entry/davi-kalam-16
https://portfolium.com/entry/davi-kalam-15
https://portfolium.com/entry/davi-kalam-14
https://portfolium.com/entry/davi-kalam-13
https://portfolium.com/entry/davi-kalam-12
portfolium.com/entry/top-10-123
https://portfolium.com/entry/top-10-124
https://portfolium.com/entry/top-10-125
https://portfolium.com/entry/8-8
https://portfolium.com/entry/top-7-100-10
https://portfolium.com/entry/10-99
https://portfolium.com/entry/10-2070
https://portfolium.com/entry/10-2071
https://portfolium.com/entry/10-2072
https://portfolium.com/entry/10-2073
https://portfolium.com/entry/10-2074
https://portfolium.com/entry/10-2075
https://portfolium.com/entry/10-2076
https://portfolium.com/entry/10-2077
https://portfolium.com/entry/10-2078
https://portfolium.com/entry/10-2079
https://portfolium.com/entry/10-2080
https://portfolium.com/entry/10-2081
https://portfolium.com/entry/10-2082
https://portfolium.com/entry/10-2083
https://portfolium.com/entry/10-2084
https://portfolium.com/entry/10-2085
https://portfolium.com/entry/10-2086
https://portfolium.com/entry/10-2087
https://portfolium.com/entry/10-2088
https://portfolium.com/entry/10-2089
https://portfolium.com/entry/ufabet-2025
https://portfolium.com/entry/ufabet-2025-1
https://portfolium.com/entry/ufabet-2025-2
https://portfolium.com/entry/ufabet-2025-3
https://portfolium.com/entry/ufabet-2025-4
https://portfolium.com/entry/ufabet-2025-5
https://portfolium.com/entry/ufabet-2025-6
https://portfolium.com/entry/ufabet-2025-7
https://portfolium.com/entry/ufabet-2025-8
https://portfolium.com/entry/ufabet-2025-9
https://portfolium.com/entry/10-1-1
https://portfolium.com/entry/top-5-lineab555-4
https://portfolium.com/entry/top-6-lineab555-3
https://portfolium.com/entry/top-7-lineab555-3
https://portfolium.com/entry/top-8-lineab555-2
https://portfolium.com/entry/16-1
https://portfolium.com/entry/17-1
https://portfolium.com/entry/14-1-1
https://portfolium.com/entry/13-1
https://portfolium.com/entry/12-1
https://portfolium.com/entry/4-1-1
https://portfolium.com/entry/3-1-1
https://portfolium.com/entry/7-1-1
https://portfolium.com/entry/5-1-1
https://portfolium.com/entry/6-1-1
https://portfolium.com/entry/top-12-1
https://portfolium.com/entry/top-13-1
https://portfolium.com/entry/top-14-1
https://portfolium.com/entry/top-16-1
https://portfolium.com/entry/top-17-1
https://portfolium.com/entry/top-11-1-1
https://portfolium.com/entry/top-12-1-1
https://portfolium.com/entry/top-13-1-1
https://portfolium.com/entry/top-10-1-26
https://portfolium.com/entry/top-17-1-1
https://portfolium.com/entry/top-1-1
https://portfolium.com/entry/top-2-1
https://portfolium.com/entry/top-3-1
https://portfolium.com/entry/top-4-1-1
https://portfolium.com/entry/top-5-1-2
https://portfolium.com/entry/12-1-1
https://portfolium.com/entry/13-1-1
https://portfolium.com/entry/17-1-1
https://portfolium.com/entry/18-1
https://portfolium.com/entry/16-1-1
https://portfolium.com/entry/10-ab555-9
https://portfolium.com/entry/12-ab555-5
https://portfolium.com/entry/10-ab555-8
https://portfolium.com/entry/11-ab555-3
https://portfolium.com/entry/13-ab555-3
https://portfolium.com/entry/12-ab555-4
https://portfolium.com/entry/9-ab555-6
https://portfolium.com/entry/8-ab555-4
https://portfolium.com/entry/17-ab555-3
https://portfolium.com/entry/16-ab555-3
https://portfolium.com/entry/7-ab555-7
https://portfolium.com/entry/6-ab555-5
https://portfolium.com/entry/14-ab555-6
https://portfolium.com/entry/15-ab555-4
https://portfolium.com/entry/14-ab555-7
https://portfolium.com/entry/20-ab555-2
https://portfolium.com/entry/top-2-ab555-4
https://portfolium.com/entry/top-3-ab555-5
https://portfolium.com/entry/21-ab555-2
https://portfolium.com/entry/22-ab555-2
https://portfolium.com/entry/11-ab555-4
https://portfolium.com/entry/16-ab555-4
https://portfolium.com/entry/24-ab555-2
https://portfolium.com/entry/23-ab555-2
https://portfolium.com/entry/13-ab555-4
https://portfolium.com/entry/17-ab555-4
https://portfolium.com/entry/top-4-ab555-5
https://portfolium.com/entry/top-5-ab555-15
https://portfolium.com/entry/top-1-ab555-3
https://portfolium.com/entry/top-2-ab555-5
https://portfolium.com/entry/top-3-ab555-6
https://portfolium.com/entry/top-6-ab555-7
https://portfolium.com/entry/top-7-ab555-8
https://portfolium.com/entry/top-8-ab555-9
https://portfolium.com/entry/top-9-ab555-11
https://portfolium.com/entry/top-10-100-2
https://portfolium.com/entry/top-10-100-3
https://portfolium.com/entry/top-10-100-4
https://portfolium.com/entry/top-10-100-5
https://portfolium.com/entry/top-10-100-6
https://portfolium.com/entry/top-10-100-7
https://portfolium.com/entry/top-10-100-8
https://portfolium.com/entry/top-10-100-9
https://portfolium.com/entry/top-10-100-10
https://portfolium.com/entry/top-10-100-11
https://portfolium.com/entry/10-ab555-10
https://portfolium.com/entry/10-ab555-11
https://portfolium.com/entry/10-ab555-12
https://portfolium.com/entry/10-ab555-13
https://portfolium.com/entry/10-ab555-14
https://portfolium.com/entry/10-ab555-15
https://portfolium.com/entry/10-ab555-16
https://portfolium.com/entry/10-ab555-17
https://portfolium.com/entry/10-ab555-18
https://portfolium.com/entry/10-ab555-19
https://portfolium.com/entry/6-ab555-8
https://portfolium.com/entry/6-ab555-9
https://portfolium.com/entry/6-ab555-10
https://portfolium.com/entry/6-ab555-11
https://portfolium.com/entry/6-ab555-12
https://portfolium.com/entry/6-ab555-13
https://portfolium.com/entry/6-ab555-14
https://portfolium.com/entry/6-ab555-15
https://portfolium.com/entry/6-ab555-16
https://portfolium.com/entry/6-ab555-17
https://portfolium.com/entry/top-10-ab555-16
https://portfolium.com/entry/top-10-ab555-17
https://portfolium.com/entry/top-12-ab555-1
https://portfolium.com/entry/top-13-ab555-5
https://portfolium.com/entry/top-13-ab555-6
https://portfolium.com/entry/top-14-ab555
https://portfolium.com/entry/top-15-ab555-1
https://portfolium.com/entry/top-16-ab555
https://portfolium.com/entry/top-17-ab555
https://portfolium.com/entry/top-18-ab555
https://portfolium.com/entry/top-10-ab555-18
https://portfolium.com/entry/top-9-ab555-12
https://portfolium.com/entry/top-8-ab555-10
https://portfolium.com/entry/top-7-ab555-9
https://portfolium.com/entry/top-6-ab555-8
https://portfolium.com/entry/top-1-ab555-4
https://portfolium.com/entry/top-2-ab555-6
https://portfolium.com/entry/top-5-ab555-16
https://portfolium.com/entry/top-4-ab555-6
https://portfolium.com/entry/top-3-ab555-7
https://portfolium.com/entry/top-10-ab555-19
https://portfolium.com/entry/top-18-ab555-1
https://portfolium.com/entry/top-6-ab555-9
https://portfolium.com/entry/top-7-ab555-10
https://portfolium.com/entry/top-8-ab555-11
https://portfolium.com/entry/top-14-ab555-1
https://portfolium.com/entry/top-13-ab555-7
https://portfolium.com/entry/top-12-ab555-2
https://portfolium.com/entry/top-10-ab555-20
https://portfolium.com/entry/top-11-ab555-2
https://portfolium.com/entry/top-10-100-1
https://portfolium.com/entry/top-8-100
https://portfolium.com/entry/top-9-100-10
https://portfolium.com/entry/top-7-100-11
https://portfolium.com/entry/top-5-100
https://portfolium.com/entry/top-6-100
https://portfolium.com/entry/top-4-100
https://portfolium.com/entry/top-3-100
https://portfolium.com/entry/top-2-100
https://portfolium.com/entry/17-ab555-5
https://portfolium.com/entry/18-ab555-2
https://portfolium.com/entry/19-ab555-2
https://portfolium.com/entry/14-ab555-8
https://portfolium.com/entry/15-ab555-5
https://portfolium.com/entry/16-ab555-5
https://portfolium.com/entry/13-ab555-5
https://portfolium.com/entry/12-ab555-6
https://portfolium.com/entry/11-ab555-5
https://portfolium.com/entry/15-ab555-7
https://portfolium.com/entry/15-ab555-7
https://portfolium.com/entry/16-ab555-6
https://portfolium.com/entry/6-ab555-7
https://portfolium.com/entry/13-ab555-6
https://portfolium.com/entry/12-ab555-7
https://portfolium.com/entry/14-ab555-9
https://portfolium.com/entry/15-ab555-6
https://portfolium.com/entry/6-ab555-6
https://portfolium.com/entry/5-ab555-5
https://portfolium.com/entry/4-ab555-6
https://portfolium.com/entry/7-ab555-8
https://portfolium.com/entry/10-2090
https://portfolium.com/entry/9-92041
https://portfolium.com/entry/duyhung-truong-8
https://portfolium.com/entry/duyhung-truong-7
https://portfolium.com/entry/vionariri-caniago-30129725
https://portfolium.com/entry/emilianojasuo-caniago-30129725
https://portfolium.com/entry/melaniapuspita-caniago-30129725
https://portfolium.com/entry/jokopurwotok-caniago-30129725
https://portfolium.com/entry/mariahasta-caniago-30129725
https://portfolium.com/entry/dandiimanuel-caniago-30129725
https://portfolium.com/entry/aurayunati-caniago-30129725
https://portfolium.com/entry/cintaanindira-caniag-30129725
https://portfolium.com/entry/kurnianto-caniago-30129725
https://portfolium.com/entry/kusumariana-caniago-30129725
https://portfolium.com/entry/24-900-16
https://portfolium.com/entry/24-900-17
https://portfolium.com/entry/24-900-18
https://portfolium.com/entry/24-900-19
https://portfolium.com/entry/24-900-20
https://portfolium.com/entry/24-900-21
https://portfolium.com/entry/24-900-22
https://portfolium.com/entry/24-900-23
https://portfolium.com/entry/24-900-24
https://portfolium.com/entry/24-900-25
https://portfolium.com/entry/top-6-101
https://portfolium.com/entry/top-6-102
https://portfolium.com/entry/top-6-103
https://portfolium.com/entry/top-6-104
https://portfolium.com/entry/top-6-105
https://portfolium.com/entry/top-6-106
https://portfolium.com/entry/top-6-107
https://portfolium.com/entry/top-6-108
https://portfolium.com/entry/top-6-109
https://portfolium.com/entry/top-6-110
https://portfolium.com/entry/top-7-ab555-1-1
https://portfolium.com/entry/top-6-ab555-1-1
https://portfolium.com/entry/top-5-ab555-1-1
https://portfolium.com/entry/top-8-ab555-1-1
https://portfolium.com/entry/top-9-ab555-1-2
https://portfolium.com/entry/top-14-ab555-1-1
https://portfolium.com/entry/top-13-ab555-1-1
https://portfolium.com/entry/top-12-ab555-1-1
https://portfolium.com/entry/top-11-ab555-1-1
https://portfolium.com/entry/top-10-ab555-1-1
https://portfolium.com/entry/top-15-ab555-1-1
https://portfolium.com/entry/top-16-ab555-1
https://portfolium.com/entry/top-17-ab555-1
https://portfolium.com/entry/top-4-ab555-1-2
https://portfolium.com/entry/top-18-ab555-1-1
https://portfolium.com/entry/top-7-ab555-1-2
https://portfolium.com/entry/top-12-ab555-1-2
https://portfolium.com/entry/top-13-ab555-1-2
https://portfolium.com/entry/top-14-ab555-1-2
https://portfolium.com/entry/top-10-ab555-1-2
https://portfolium.com/entry/top-5-ab555-1-2
https://portfolium.com/entry/top-4-ab555-1-3
https://portfolium.com/entry/top-3-ab555-1-2
https://portfolium.com/entry/top-2-ab555-1-2
https://portfolium.com/entry/top-1-ab555-1-1
https://portfolium.com/entry/top-7-ab555-1-3
https://portfolium.com/entry/top-8-ab555-1-2
https://portfolium.com/entry/top-9-ab555-1-3
https://portfolium.com/entry/top-10-ab555-1-3
https://portfolium.com/entry/top-12-ab555-1-3
https://portfolium.com/entry/top-10-ab555-1-4
https://portfolium.com/entry/top-7-ab555-1-4
https://portfolium.com/entry/top-5-ab555-1-3
https://portfolium.com/entry/top-6-ab555-1-2
https://portfolium.com/entry/top-16-ab555-1-1
https://portfolium.com/entry/top-10-ab555-21
https://portfolium.com/entry/13-1-2
https://portfolium.com/entry/20-ab555-3
https://portfolium.com/entry/16-ab555-7
https://portfolium.com/entry/17-ab555-6
https://portfolium.com/entry/14-ab555-10
https://portfolium.com/entry/15-ab555-8
https://portfolium.com/entry/12-ab555-8
https://portfolium.com/entry/11-ab555-7
https://portfolium.com/entry/10-ab555-20
https://portfolium.com/entry/24-ab555-3
https://portfolium.com/entry/top-7-ab555-11
https://portfolium.com/entry/8-ab555-5
https://portfolium.com/entry/9-ab555-7
https://portfolium.com/entry/7-ab555-9
https://portfolium.com/entry/21-ab555-3
https://portfolium.com/entry/22-ab555-3
https://portfolium.com/entry/23-ab555-3
https://portfolium.com/entry/4-1-2
https://portfolium.com/entry/18-1-1
https://portfolium.com/entry/17-1-2
https://portfolium.com/entry/16-1-2
https://portfolium.com/entry/15-1
https://portfolium.com/entry/14-1-2
https://portfolium.com/entry/13-1-3
https://portfolium.com/entry/12-1-2
https://portfolium.com/entry/11-1-5
https://portfolium.com/entry/5-1-2
https://portfolium.com/entry/6-1-2
https://portfolium.com/entry/7-1-2
https://portfolium.com/entry/8-1-1
https://portfolium.com/entry/9-1-1
https://portfolium.com/entry/20-1
https://portfolium.com/entry/22-1
https://portfolium.com/entry/23-1
https://portfolium.com/entry/top-10-100-15
https://portfolium.com/entry/top-9-100-13
https://portfolium.com/entry/top-9-100-14
https://portfolium.com/entry/top-8-100-3
https://portfolium.com/entry/top-8-100-4
https://portfolium.com/entry/top-7-100-14
https://portfolium.com/entry/top-7-100-15
https://portfolium.com/entry/top-6-100-3
https://portfolium.com/entry/top-6-100-4
https://portfolium.com/entry/top-5-100-3
https://portfolium.com/entry/top-5-100-4
https://portfolium.com/entry/top-9-100-12
https://portfolium.com/entry/top-10-100-14
https://portfolium.com/entry/top-7-100-13
https://portfolium.com/entry/top-8-100-2
https://portfolium.com/entry/top-5-100-2
https://portfolium.com/entry/top-6-100-2
https://portfolium.com/entry/top-10-100-12
https://portfolium.com/entry/top-10-100-13
https://portfolium.com/entry/top-8-100-1
https://portfolium.com/entry/top-9-100-11
https://portfolium.com/entry/top-6-100-1
https://portfolium.com/entry/top-7-100-12
https://portfolium.com/entry/top-4-100-1
https://portfolium.com/entry/top-5-100-1
https://portfolium.com/entry/top-10-134
https://portfolium.com/entry/top-10-135
https://portfolium.com/entry/top-10-132
https://portfolium.com/entry/top-10-133
https://portfolium.com/entry/top-10-130
https://portfolium.com/entry/top-10-131
https://portfolium.com/entry/top-10-128
https://portfolium.com/entry/top-10-129
https://portfolium.com/entry/top-10-126
https://portfolium.com/entry/top-10-127
https://portfolium.com/entry/top-6-pg
https://portfolium.com/entry/top-7-pg
https://portfolium.com/entry/top-8-pg
https://portfolium.com/entry/top-9-pg
https://portfolium.com/entry/top-5-pg
https://portfolium.com/entry/top-1-pg
https://portfolium.com/entry/top-2-pg
https://portfolium.com/entry/top-3-pg
https://portfolium.com/entry/top-4-pg
https://portfolium.com/entry/top-10-pg
https://portfolium.com/entry/14-pg-2
https://portfolium.com/entry/13-pg-3
https://portfolium.com/entry/12-pg
https://portfolium.com/entry/11-pg
https://portfolium.com/entry/10-pg-3
https://portfolium.com/entry/top-16-pg
https://portfolium.com/entry/top-15-pg
https://portfolium.com/entry/top-14-pg
https://portfolium.com/entry/top-13-pg
https://portfolium.com/entry/top-12-pg
portfolium.com/entry/13-pg-4
https://portfolium.com/entry/19-pg-2
https://portfolium.com/entry/10-pg-4
https://portfolium.com/entry/18-pg
https://portfolium.com/entry/17-pg-2
https://portfolium.com/entry/1-pg
https://portfolium.com/entry/2-pg
https://portfolium.com/entry/3-pg
https://portfolium.com/entry/4-pg
https://portfolium.com/entry/6-pg-3
https://portfolium.com/entry/top-5-pg-1
https://portfolium.com/entry/top-6-pg-1
https://portfolium.com/entry/top-7-pg-1
https://portfolium.com/entry/top-8-pg-1
https://portfolium.com/entry/top-9-pg-1
https://portfolium.com/entry/7-1-ab555
https://portfolium.com/entry/8-1-ab555
https://portfolium.com/entry/9-1-ab555
https://portfolium.com/entry/top-5-ab555-17
https://portfolium.com/entry/top-9-ab555-13
https://portfolium.com/entry/top-10-ab555-22
https://portfolium.com/entry/13-1-ab555-1
https://portfolium.com/entry/14-1-ab555-1
https://portfolium.com/entry/15-1-ab555
https://portfolium.com/entry/13-1-ab555
https://portfolium.com/entry/12-1-ab555
https://portfolium.com/entry/11-1-ab555
https://portfolium.com/entry/17-ab555-7
https://portfolium.com/entry/10-api
https://portfolium.com/entry/7-api-1
https://portfolium.com/entry/4-api
https://portfolium.com/entry/5-ab555-6
https://portfolium.com/entry/9-ab555-8
https://portfolium.com/entry/6-ab555-18
https://portfolium.com/entry/11-ab555-8
https://portfolium.com/entry/top-4-ab555-7
https://portfolium.com/entry/top-3-ab555-8
https://portfolium.com/entry/top-2-ab555-7
https://portfolium.com/entry/13-ab555-7
https://portfolium.com/entry/14-ab555-11
https://portfolium.com/entry/16-ab555-9
https://portfolium.com/entry/15-ab555-9
https://portfolium.com/entry/4-pg-1-1
https://portfolium.com/entry/6-pg-1-2
https://portfolium.com/entry/7-pg-1-3
https://portfolium.com/entry/10-ab555-21
https://portfolium.com/entry/top-6-ab555-10
https://portfolium.com/entry/top-7-ab555-12
https://portfolium.com/entry/top-8-ab555-12
https://portfolium.com/entry/16-ab555-8
https://portfolium.com/entry/24-900-26
https://portfolium.com/entry/24-900-27
https://portfolium.com/entry/24-900-28
https://portfolium.com/entry/24-900-29
https://portfolium.com/entry/24-900-30
https://portfolium.com/entry/24-900-31
https://portfolium.com/entry/24-900-32
https://portfolium.com/entry/24-900-33
https://portfolium.com/entry/24-900-34
https://portfolium.com/entry/24-900-35
https://portfolium.com/entry/top-5-24-900
https://portfolium.com/entry/top-5-24-900-1
https://portfolium.com/entry/top-6-24-900
https://portfolium.com/entry/top-6-24-900-1
https://portfolium.com/entry/top-7-24-900
https://portfolium.com/entry/top-7-24-900-1
https://portfolium.com/entry/top-8-24-900
https://portfolium.com/entry/top-8-24-900-1
https://portfolium.com/entry/top-9-24-900
https://portfolium.com/entry/top-9-24-900-1
https://portfolium.com/entry/5-24
https://portfolium.com/entry/5-24-25
https://portfolium.com/entry/6-24-1
https://portfolium.com/entry/6-24-2
https://portfolium.com/entry/7-24
https://portfolium.com/entry/7-24-1
https://portfolium.com/entry/8-24
https://portfolium.com/entry/8-24-1
https://portfolium.com/entry/9-24
https://portfolium.com/entry/9-24-1
https://portfolium.com/entry/10-24-3
https://portfolium.com/entry/10-24-6
https://portfolium.com/entry/10-24-7
https://portfolium.com/entry/10-24-4
https://portfolium.com/entry/10-24-5
https://portfolium.com/entry/top-5-24
https://portfolium.com/entry/top-10-24-1
https://portfolium.com/entry/top-12-24
https://portfolium.com/entry/6-24-5
https://portfolium.com/entry/top-4-24-2
https://portfolium.com/entry/top-2-24-1
https://portfolium.com/entry/top-1-24-1
https://portfolium.com/entry/top-3-24-3
https://portfolium.com/entry/10-24-2
https://portfolium.com/entry/8-24-4
https://portfolium.com/entry/5-24-28
https://portfolium.com/entry/top-7-24-902
https://portfolium.com/entry/top-12-24-1
https://portfolium.com/entry/top-13-24
https://portfolium.com/entry/top-6-24-902
https://portfolium.com/entry/top-17-24
https://portfolium.com/entry/top-10-24-4
https://portfolium.com/entry/top-13-24-1
https://portfolium.com/entry/16-24
https://portfolium.com/entry/4-24-1
https://portfolium.com/entry/top-3-24-1
https://portfolium.com/entry/top-4-24-1
https://portfolium.com/entry/top-10-24-3
https://portfolium.com/entry/top-6-24-901
https://portfolium.com/entry/top-7-24-901
https://portfolium.com/entry/top-8-24-901
https://portfolium.com/entry/12-24-1
https://portfolium.com/entry/13-24-1
https://portfolium.com/entry/10-24-1
https://portfolium.com/entry/5-24-27
https://portfolium.com/entry/8-24-3
https://portfolium.com/entry/7-24-5
https://portfolium.com/entry/top-3-24-2
https://portfolium.com/entry/6-24-3
https://portfolium.com/entry/7-24-2
https://portfolium.com/entry/6-24-4
https://portfolium.com/entry/4-24
https://portfolium.com/entry/5-24-26
https://portfolium.com/entry/7-24-3
https://portfolium.com/entry/8-24-2
https://portfolium.com/entry/9-24-2
https://portfolium.com/entry/7-24-4
https://portfolium.com/entry/10-24
https://portfolium.com/entry/top-1-24
https://portfolium.com/entry/top-2-24
https://portfolium.com/entry/top-3-24
https://portfolium.com/entry/top-4-24
https://portfolium.com/entry/top-5-24-901
https://portfolium.com/entry/top-6-24
https://portfolium.com/entry/top-7-24
https://portfolium.com/entry/top-8-24
https://portfolium.com/entry/top-9-24
https://portfolium.com/entry/top-10-24-2
https://portfolium.com/entry/11-24
https://portfolium.com/entry/12-24
https://portfolium.com/entry/13-24
https://portfolium.com/entry/14-24
https://portfolium.com/entry/13-ab555-24
https://portfolium.com/entry/14-ab555-24
https://portfolium.com/entry/15-ab555-24
https://portfolium.com/entry/16-ab555-24
https://portfolium.com/entry/7-ab555-24
https://portfolium.com/entry/4-ab555-24
https://portfolium.com/entry/5-ab555-24
https://portfolium.com/entry/6-ab555-24
https://portfolium.com/entry/8-ab555-24
https://portfolium.com/entry/9-ab555-24
https://portfolium.com/entry/10-ab555-24
https://portfolium.com/entry/11-ab555-24
https://portfolium.com/entry/12-ab555-24
https://portfolium.com/entry/top-6-ab555
https://portfolium.com/entry/10-2026
https://portfolium.com/entry/24-900
https://portfolium.com/entry/xiu-magi-24